User login
Polycyclic Scaly Eruption
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
A 9-year-old boy presented to the dermatology clinic with a scaly eruption distributed throughout the body that had been present since birth. He had been diagnosed with atopic dermatitis by multiple dermatologists prior to the current presentation and had been treated with various topical steroids with minimal improvement. He had no family history of similar eruptions and no personal history of asthma or allergies. Physical examination revealed erythematous, serpiginous, polycyclic plaques with peripheral, double-edged scaling. Decreased hair density of the lateral eyebrows also was observed.

Telangiectases on the Cheeks and Nose
The Diagnosis: Hereditary Hemorrhagic Telangiectasia
Physical examination of our patient revealed multiple fine punctate telangiectases on the bilateral cheeks and the dorsum of the nose. Further examination revealed matlike telangiectases on the distal aspect of the tongue (Figure), buccal mucosa, palms, and fingers. Since diagnosis he has experienced several episodes of severe gastrointestinal tract bleeding. He underwent an esophagogastroduodenoscopy and was found to have bleeding gastric antral vascular ectasia that was treated with argon plasma coagulation. One year later he had a second esophagogastroduodenoscopy performed because of heme-positive stools and was found to have additional bleeding vascular ectasia that was treated again with argon plasma coagulation.
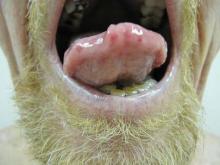
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal-dominant disorder that is characterized by mucocutaneous and visceral telangiectases, recurrent hemorrhages, and a familial occurrence.1 In North America, the overall incidence is approximately 1 per 5000 to 10,000 individuals per year.2 Although this disorder generally has an autosomal-dominant transmission, approximately 20% of cases do not have a familial component.2
Clinically these patients most commonly present with a recurrent episode of epistaxis that occurs within the first 2 decades of life. Shortly after the onset of these recurrent episodes, patients begin to develop punctate or splinterlike telangiectases on the lips, oral mucosa, upper extremities, nail beds, and trunk. These cutaneous telangiectases are a cosmetic problem and can sometimes cause hemorrhage, especially from the tongue and fingers.1 The serious complications of HHT arise from internal organ involvement. Gastrointestinal telangiectases and arteriovenous malformations (AVMs) can result in acute gastrointestinal hemorrhages or iron deficiency anemia in approximately 16% of patients. Central nervous system AVMs result in migraines, brain abscesses, paraparesis, ischemia, strokes, transient ischemic attacks, seizures, and both intracerebral and subarachnoid hemorrhage. Pulmonary AVMs can cause a right-to-left shunt, leading to hypoxemia and embolic events due to the bypass of the lungs, which act as a filtering capillary bed.3
Genetic linkage analysis has revealed 2 genes that are responsible for HHT. The first gene, ENG, encodes endoglin and is found on band 9q33-34.3 The second gene, ALK1, encodes activin receptorlike kinase 1 and is found on band 12q11-12. Both of these gene products are involved with vascular remodeling. They are integral membrane glycoproteins mainly expressed on vascular endothelial cells and act as surface receptors for transforming growth factor b. Mutations in ALK1 are associated with a generally more benign course, whereas mutations in ENG more commonly have pulmonary AVMs.3
The diagnosis of HHT is established if 3 of the following features are present: (1) epistaxis (ie, spontaneous recurrent nosebleeds); (2) telangiectases in characteristic sites (ie, oral cavity, lips, nose, fingers); (3) visceral lesions, such as gastrointestinal telangiectases (with or without bleeding), pulmonary AVM, hepatic AVM, cerebral AVM, or spinal AVM; (4) family history (ie, a first-degree relative with HHT).3 Similar diseases with telangiectases should be considered in the differential diagnosis including CREST syndrome (characterized by calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia), generalized essential telangiectasia, and ataxia telangiectasia.2
Patients diagnosed with HHT who have a family history of the disease should be evaluated for pulmonary AVM through chest computed tomography and pulmonary angiography because they are at the highest risk. Magnetic resonance imaging is useful to rule out central nervous system involvement. Treatment of cutaneous telangiectases consists of electrocauterization; sclerotherapy; and a variety of lasers and light sources including the Nd:YAG laser, intense pulsed light, argon laser, or pulsed dye laser.1,2,4 An extremely useful resource for patients with HHT and family members is the HHT Foundation (http://curehht.org).
1. Fernández-Jorge B, Del Pozo Losada J, Paradela S, et al. Treatment of cutaneous and mucosal telangiectases in hereditary hemorrhagic telangiectasia: report of three cases. J Cosmet Laser Ther. 2007;9:29-33.
2. Lee HE, Sagong C, Yeo KY, et al. A case of hereditary hemorrhagic telangiectasia. Ann Dermatol. 2009;21:206-208.
3. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations. part II: associated syndromes. J Am Acad Dermatol. 2007;56:541-564.
4. Sato Y, Takayama T, Takahari D, et al. Successful treatment for gastro-intestinal bleeding of Osler-Weber-Rendu disease by argon plasma coagulation using double-balloon enteroscopy. Endoscopy. 2008;40(suppl 2):E228-E229.
The Diagnosis: Hereditary Hemorrhagic Telangiectasia
Physical examination of our patient revealed multiple fine punctate telangiectases on the bilateral cheeks and the dorsum of the nose. Further examination revealed matlike telangiectases on the distal aspect of the tongue (Figure), buccal mucosa, palms, and fingers. Since diagnosis he has experienced several episodes of severe gastrointestinal tract bleeding. He underwent an esophagogastroduodenoscopy and was found to have bleeding gastric antral vascular ectasia that was treated with argon plasma coagulation. One year later he had a second esophagogastroduodenoscopy performed because of heme-positive stools and was found to have additional bleeding vascular ectasia that was treated again with argon plasma coagulation.

Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal-dominant disorder that is characterized by mucocutaneous and visceral telangiectases, recurrent hemorrhages, and a familial occurrence.1 In North America, the overall incidence is approximately 1 per 5000 to 10,000 individuals per year.2 Although this disorder generally has an autosomal-dominant transmission, approximately 20% of cases do not have a familial component.2
Clinically these patients most commonly present with a recurrent episode of epistaxis that occurs within the first 2 decades of life. Shortly after the onset of these recurrent episodes, patients begin to develop punctate or splinterlike telangiectases on the lips, oral mucosa, upper extremities, nail beds, and trunk. These cutaneous telangiectases are a cosmetic problem and can sometimes cause hemorrhage, especially from the tongue and fingers.1 The serious complications of HHT arise from internal organ involvement. Gastrointestinal telangiectases and arteriovenous malformations (AVMs) can result in acute gastrointestinal hemorrhages or iron deficiency anemia in approximately 16% of patients. Central nervous system AVMs result in migraines, brain abscesses, paraparesis, ischemia, strokes, transient ischemic attacks, seizures, and both intracerebral and subarachnoid hemorrhage. Pulmonary AVMs can cause a right-to-left shunt, leading to hypoxemia and embolic events due to the bypass of the lungs, which act as a filtering capillary bed.3
Genetic linkage analysis has revealed 2 genes that are responsible for HHT. The first gene, ENG, encodes endoglin and is found on band 9q33-34.3 The second gene, ALK1, encodes activin receptorlike kinase 1 and is found on band 12q11-12. Both of these gene products are involved with vascular remodeling. They are integral membrane glycoproteins mainly expressed on vascular endothelial cells and act as surface receptors for transforming growth factor b. Mutations in ALK1 are associated with a generally more benign course, whereas mutations in ENG more commonly have pulmonary AVMs.3
The diagnosis of HHT is established if 3 of the following features are present: (1) epistaxis (ie, spontaneous recurrent nosebleeds); (2) telangiectases in characteristic sites (ie, oral cavity, lips, nose, fingers); (3) visceral lesions, such as gastrointestinal telangiectases (with or without bleeding), pulmonary AVM, hepatic AVM, cerebral AVM, or spinal AVM; (4) family history (ie, a first-degree relative with HHT).3 Similar diseases with telangiectases should be considered in the differential diagnosis including CREST syndrome (characterized by calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia), generalized essential telangiectasia, and ataxia telangiectasia.2
Patients diagnosed with HHT who have a family history of the disease should be evaluated for pulmonary AVM through chest computed tomography and pulmonary angiography because they are at the highest risk. Magnetic resonance imaging is useful to rule out central nervous system involvement. Treatment of cutaneous telangiectases consists of electrocauterization; sclerotherapy; and a variety of lasers and light sources including the Nd:YAG laser, intense pulsed light, argon laser, or pulsed dye laser.1,2,4 An extremely useful resource for patients with HHT and family members is the HHT Foundation (http://curehht.org).
The Diagnosis: Hereditary Hemorrhagic Telangiectasia
Physical examination of our patient revealed multiple fine punctate telangiectases on the bilateral cheeks and the dorsum of the nose. Further examination revealed matlike telangiectases on the distal aspect of the tongue (Figure), buccal mucosa, palms, and fingers. Since diagnosis he has experienced several episodes of severe gastrointestinal tract bleeding. He underwent an esophagogastroduodenoscopy and was found to have bleeding gastric antral vascular ectasia that was treated with argon plasma coagulation. One year later he had a second esophagogastroduodenoscopy performed because of heme-positive stools and was found to have additional bleeding vascular ectasia that was treated again with argon plasma coagulation.

Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal-dominant disorder that is characterized by mucocutaneous and visceral telangiectases, recurrent hemorrhages, and a familial occurrence.1 In North America, the overall incidence is approximately 1 per 5000 to 10,000 individuals per year.2 Although this disorder generally has an autosomal-dominant transmission, approximately 20% of cases do not have a familial component.2
Clinically these patients most commonly present with a recurrent episode of epistaxis that occurs within the first 2 decades of life. Shortly after the onset of these recurrent episodes, patients begin to develop punctate or splinterlike telangiectases on the lips, oral mucosa, upper extremities, nail beds, and trunk. These cutaneous telangiectases are a cosmetic problem and can sometimes cause hemorrhage, especially from the tongue and fingers.1 The serious complications of HHT arise from internal organ involvement. Gastrointestinal telangiectases and arteriovenous malformations (AVMs) can result in acute gastrointestinal hemorrhages or iron deficiency anemia in approximately 16% of patients. Central nervous system AVMs result in migraines, brain abscesses, paraparesis, ischemia, strokes, transient ischemic attacks, seizures, and both intracerebral and subarachnoid hemorrhage. Pulmonary AVMs can cause a right-to-left shunt, leading to hypoxemia and embolic events due to the bypass of the lungs, which act as a filtering capillary bed.3
Genetic linkage analysis has revealed 2 genes that are responsible for HHT. The first gene, ENG, encodes endoglin and is found on band 9q33-34.3 The second gene, ALK1, encodes activin receptorlike kinase 1 and is found on band 12q11-12. Both of these gene products are involved with vascular remodeling. They are integral membrane glycoproteins mainly expressed on vascular endothelial cells and act as surface receptors for transforming growth factor b. Mutations in ALK1 are associated with a generally more benign course, whereas mutations in ENG more commonly have pulmonary AVMs.3
The diagnosis of HHT is established if 3 of the following features are present: (1) epistaxis (ie, spontaneous recurrent nosebleeds); (2) telangiectases in characteristic sites (ie, oral cavity, lips, nose, fingers); (3) visceral lesions, such as gastrointestinal telangiectases (with or without bleeding), pulmonary AVM, hepatic AVM, cerebral AVM, or spinal AVM; (4) family history (ie, a first-degree relative with HHT).3 Similar diseases with telangiectases should be considered in the differential diagnosis including CREST syndrome (characterized by calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia), generalized essential telangiectasia, and ataxia telangiectasia.2
Patients diagnosed with HHT who have a family history of the disease should be evaluated for pulmonary AVM through chest computed tomography and pulmonary angiography because they are at the highest risk. Magnetic resonance imaging is useful to rule out central nervous system involvement. Treatment of cutaneous telangiectases consists of electrocauterization; sclerotherapy; and a variety of lasers and light sources including the Nd:YAG laser, intense pulsed light, argon laser, or pulsed dye laser.1,2,4 An extremely useful resource for patients with HHT and family members is the HHT Foundation (http://curehht.org).
1. Fernández-Jorge B, Del Pozo Losada J, Paradela S, et al. Treatment of cutaneous and mucosal telangiectases in hereditary hemorrhagic telangiectasia: report of three cases. J Cosmet Laser Ther. 2007;9:29-33.
2. Lee HE, Sagong C, Yeo KY, et al. A case of hereditary hemorrhagic telangiectasia. Ann Dermatol. 2009;21:206-208.
3. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations. part II: associated syndromes. J Am Acad Dermatol. 2007;56:541-564.
4. Sato Y, Takayama T, Takahari D, et al. Successful treatment for gastro-intestinal bleeding of Osler-Weber-Rendu disease by argon plasma coagulation using double-balloon enteroscopy. Endoscopy. 2008;40(suppl 2):E228-E229.
1. Fernández-Jorge B, Del Pozo Losada J, Paradela S, et al. Treatment of cutaneous and mucosal telangiectases in hereditary hemorrhagic telangiectasia: report of three cases. J Cosmet Laser Ther. 2007;9:29-33.
2. Lee HE, Sagong C, Yeo KY, et al. A case of hereditary hemorrhagic telangiectasia. Ann Dermatol. 2009;21:206-208.
3. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations. part II: associated syndromes. J Am Acad Dermatol. 2007;56:541-564.
4. Sato Y, Takayama T, Takahari D, et al. Successful treatment for gastro-intestinal bleeding of Osler-Weber-Rendu disease by argon plasma coagulation using double-balloon enteroscopy. Endoscopy. 2008;40(suppl 2):E228-E229.

A 68-year-old man presented for evaluation of numerous telangiectases that developed on the cheeks, nose, lips, tongue, and fingers. He denied any history of gastrointestinal tract bleeding but admitted to numerous nosebleeds in the recent past. Family history indicated that his maternal grandmother died of an aneurysm, his mother died of a cerebral hemorrhage, 2 paternal cousins had hemochromatosis, and 2 brothers were healthy.

