User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
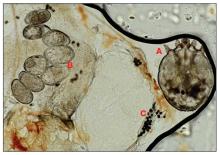
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.
The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
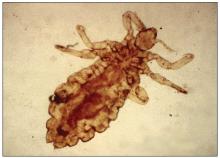
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27
Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32
Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>Strahan</fileName> <TBEID>0C02F581.SIG</TBEID> <TBUniqueIdentifier>NJ_0C02F581</TBUniqueIdentifier> <newsOrJournal>Journal</newsOrJournal> <publisherName>Frontline Medical Communications Inc.</publisherName> <storyname>Clinical Review</storyname> <articleType>1</articleType> <TBLocation>Published-CT</TBLocation> <QCDate/> <firstPublished>20240425T131253</firstPublished> <LastPublished>20240425T132642</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240425T131253</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Strahan</byline> <bylineText>Alexis G. Strahan, MD, MSN; Dirk M. Elston, MD</bylineText> <bylineFull>Strahan</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange>E16-E21</pageRange> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:"> <name/> <rightsInfo> <copyrightHolder> <name/> </copyrightHolder> <copyrightNotice/> </rightsInfo> </provider> <abstract/> <metaDescription>Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The U</metaDescription> <articlePDF>301172</articlePDF> <teaserImage/> <title>Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>2</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth>April</pubPubdateMonth> <pubPubdateDay/> <pubVolume>113</pubVolume> <pubNumber>4</pubNumber> <wireChannels/> <primaryCMSID/> <CMSIDs> <CMSID>2293</CMSID> <CMSID>2161</CMSID> </CMSIDs> <keywords> <keyword>infectious disease</keyword> <keyword> scabies</keyword> <keyword> pediculosis</keyword> </keywords> <seeAlsos/> <publications_g> <publicationData> <publicationCode>CT</publicationCode> <pubIssueName>April 2024</pubIssueName> <pubArticleType>Original Articles | 2161</pubArticleType> <pubTopics/> <pubCategories/> <pubSections> <pubSection>Original Article | 2293<pubSubsection/></pubSection> </pubSections> <journalTitle>Cutis</journalTitle> <journalFullTitle>Cutis</journalFullTitle> <copyrightStatement>Copyright 2015 Frontline Medical Communications Inc., Parsippany, NJ, USA. All rights reserved.</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">12</term> </publications> <sections> <term canonical="true">49</term> </sections> <topics> <term canonical="true">234</term> </topics> <links> <link> <itemClass qcode="ninat:composite"/> <altRep contenttype="application/pdf">images/1800270f.pdf</altRep> <description role="drol:caption"/> <description role="drol:credit"/> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis</title> <deck/> </itemMeta> <itemContent> <p class="abstract">There is a large burden of treatable dermatologic conditions in refugee populations. Parasitic infestations are particularly common when there are barriers to basic hygiene, crowded living or travel conditions, and lack of access to health care. Body lice are associated with anemia and can transmit a variety of diseases; chronic impetigo secondary to scabies is a leading cause of chronic kidney disease globally. Dermatologists have unique skills to identify skin infections, inflammatory diseases, and infestations. Appropriate dermatologic care has the potential to improve overall outcomes.</p> <p>Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.<sup>1,2</sup> The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.<sup>3</sup> In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.<sup>4</sup> </p> <p>Understanding how to care for this population begins with an accurate understanding of the term <i>refugee</i>. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.<sup>5,6</sup> <br/><br/>The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature<sup>7,8</sup> and varies from inflammatory disorders to infectious and parasitic diseases.<sup>9</sup> In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.<sup>10</sup> <br/><br/>When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.<sup>11</sup> Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.<sup>8</sup> <br/><br/>Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.<sup>12</sup> The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes. <br/><br/>Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.<sup>13</sup> This review will focus on infestations by the scabies mite (<i>Sarcoptes scabiei </i>var <i>hominis</i>)<i> </i>and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings. </p> <h3>Scabies </h3> <p>Scabies is a parasitic skin infestation caused by the 8-legged mite <i>Sarcoptes scabiei </i>var<i> hominis.</i> The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.<sup>14,15</sup> Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.<sup>16</sup> Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature. </p> <p>The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.<sup>17</sup> In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.<sup>18</sup> Scabies is more prevalent in children, with increased potential for secondary bacterial infections with <i>Streptococcus</i> and <i>Staphylococcus</i> species due to excoriation in unsanitary conditions. Secondary infection with <i>Streptococcus pyogenes</i> can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.<sup>19</sup> However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential. <br/><br/><i>Presentation—</i>The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.<sup>20</sup> The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.<sup>19</sup> Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.<sup>21</sup><i> <br/><br/></i>Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.<sup>22</sup> <br/><br/><i>Diagnosis—</i>The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.<sup>23</sup> A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy. <br/><br/><i>Treatment—</i>First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the <i>World Health Organization Model List of Essential Medications</i> and are widely available for treating providers, even in resource-limited settings.<sup>24</sup> <br/><br/>Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.<sup>25</sup> <em>Sarna</em> is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours. </p> <h3>Pediculosis </h3> <p>Pediculosis is an infestation caused by the ectoparasite <i>Pediculus humanus</i>, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).<sup>26</sup> Of the lice species, 2 require humans as hosts; one is <i>P humanus</i> and the other is <i>Pthirus pubis</i> (pubic lice). <i>Pediculus humanus </i>may be further classified into morphologies based largely on the affected area: body (<i>P humanus</i> <i>corporis)</i> or head (<i>P humanus</i> <i>capitis)</i>, both of which will be discussed.<sup>27</sup> </p> <p>Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.<sup>28</sup> Transmission of <i>P humanus capitis </i>primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; <i>P humanus corporis</i> also may be transmitted via direct contact with affected individuals or clothing. <br/><br/>Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.<sup>29</sup> Body lice are associated more often with domestic turbulence and displaced populations<sup>30</sup> in comparison to head lice, which have broad demographic variables, most often affecting females and children.<sup>28</sup> Fatty acids in adult male sebum make the scalp less hospitable to lice. <br/><br/><i>Presentation</i>—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed <i>maculae ceruleae</i> (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.<sup>31</sup> Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.<sup>32</sup> <br/><br/><i>Diagnosis—</i>The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).<sup>31</sup> <br/><br/><i>Treatment—</i>Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.<sup>33</sup> The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.<sup>34,35</sup> Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.<sup>22</sup> Topical corticosteroids or emollients may be employed for residual pruritus. <br/><br/>Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.<sup>36</sup> It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.<sup>37<br/><br/></sup>Pediculosis corporis may transmit infectious agents including <i>Bartonella quintana</i>, (trench fever, endocarditis, bacillary angiomatosis), <i>Borrelia recurrentis</i> (louse-borne relapsing fever), and <i>Rickettsia prowazekii</i> (epidemic typhus).<sup>31,38,39</sup> Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.<sup>40</sup> It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.<sup>41</sup> </p> <h3>Future Considerations </h3> <p>Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.<sup>42</sup></p> <p>Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.<sup>43</sup></p> <h3>Conclusion</h3> <p>Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.<sup>8,11,44</sup> Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.<sup>45</sup> Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.<sup>46</sup> Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.</p> <h2>References</h2> <p class="reference"> 1. Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021<br/><br/> 2. UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html<br/><br/> 3. UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts<br/><br/> 4. US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/ <br/><br/> 5. UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions<br/><br/> 6. United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees<br/><br/> 7. Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). <i>Int J Dermatol</i>. 2019;58:1341-1349. doi:10.1111/ijd.14619<br/><br/> 8. Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. <i>Dermatol Clin</i>. 2021;39:101-115. doi:10.1016/j.det.2020.08.010<br/><br/> 9. Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. <i>J Eur Acad Dermatol Venereol.</i> 2020;34:419-425. doi:10.1111/jdv.15909<br/><br/>10. Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. <i>J Infect Public Health</i>. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012<br/><br/>11. Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.<i>J Travel Med</i>. 2018;25. doi:10.1093/jtm/tay067<br/><br/>12. Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. <i>Trop Med Infect Dis</i>. 2018;4. doi:10.3390/tropicalmed4010004<br/><br/>13. NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases<br/><br/>14. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. <i>Parasit Vectors</i>. 2017;10:297. doi:10.1186/s13071-017-2234-1<br/><br/>15. Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. <i>J Am Acad Dermatol</i>. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4<br/><br/>16. Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. <i>Dermatology</i>. 2019;235:79-90. doi:10.1159/000495290<br/><br/>17. Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. <i>Lancet Infect Dis</i>. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8<br/><br/>18. Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. <i>Lancet Infect Dis</i>. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2<br/><br/>19. Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. <i>J Am Acad Dermatol</i>. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109<br/><br/>20. Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. <i>Br Med J</i>. 1942;2:1-4. doi:10.1136/bmj.2.4252.1<br/><br/>21. Walton SF. The immunology of susceptibility and resistance to scabies. <i>Parasit Immunol</i>. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x<br/><br/>22. Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. <i>J Am Acad Dermatol</i>. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110</p> <p class="reference">23. Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. <i>PLoS Negl Trop Dis</i>. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549<br/><br/>24. World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02 <br/><br/>25. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. <i>J Eur Acad Dermatol Venereol</i>. 2017;31:1248-1253. doi:10.1111/jdv.14351<br/><br/>26. Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. <i>Clin Microbiol Infect</i>. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x<br/><br/>27. Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. <i>J Med Entomol. </i>2002;39:662-666. doi:10.1603/0022-2585-39.4.662<br/><br/>28. Chosidow O. Scabies and pediculosis. <i>Lancet</i>. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1<br/><br/>29. Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). <i>Br J Dermatol</i>. 2016;174:104-112. doi:10.1111/bjd.14226<br/><br/>30. Brouqui P. Arthropod-borne diseases associated with political and social disorder. <i>Annu Rev Entomol</i>. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739<br/><br/>31. Ko CJ, Elston DM. Pediculosis. <i>J Am Acad Dermatol</i>. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4<br/><br/>32. Bloomfield D. Head lice. <i>Pediatr Rev</i>. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34<br/><br/>33. Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). <i>Fitzpatrick’s Dermatology in General Medicine</i>. McGraw Hill; 2008:2029.<br/><br/>34. Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. <i>J Infect Dis</i>. 2006;193:474-476. doi:10.1086/499279<br/><br/>35. Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. <i>JAMA Dermatol. </i>2014;150:273-279. doi:10.1001/jamadermatol.2013.6398<br/><br/>36. CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html<br/><br/>37. Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. <i>Pediatrics</i>. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746<br/><br/>38. Ohl ME, Spach DH. Bartonella quintana and urban trench fever. <i>Clin Infect Dis</i>. 2000;31:131-135. doi:10.1086/313890<br/><br/>39. Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. <i>Emerg Infect Dis</i>. 2014;20:907-908. doi:10.3201/eid2005.131242<br/><br/>40. Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. <i>JAMA Dermatol</i>. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818</p> <p class="reference">41. Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. <i>J Emergency Med</i>. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030<br/><br/>42. Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis<br/><br/>43. Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. <i>Br J Dermatology</i>. 2023;188:553-554. doi:10.1093/bjd/ljad001 <br/><br/>44. Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. <i>Int J Dermato</i>l. 2020;59:1299-1311. doi:10.1111/ijd.15063<br/><br/>45. Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. <i>Expert Rev Anti-infective Therapy. </i>2020;18:127-143. doi:10.1080/14787210.2020.1713099<br/><br/>46. Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>bio</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="disclosure">Alexis G. Strahan is from the Mercer University School of Medicine, Savannah, Georgia. Dr. Elston is from the Department of Dermatology and Dermatologic Surgery, Medical University of South Carolina, Charleston.</p> <p class="disclosure">The authors report no conflict of interest. <br/><br/>All images are in the public domain. <br/><br/>Correspondence: Alexis G. Strahan, MD, MSN, 55 Fruit St, Bartlett Hall 6R, Boston, MA 02114 (<a href="mailto:alexis.grabow.strahan@live.mercer.edu">alexis.grabow.strahan@live.mercer.edu</a>). <br/><br/><em><hl name="17868"/>Cutis. </em>2024 April;113(4):E16-E21. doi:10.12788/cutis.0999 </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>in</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="insidehead">Practice <strong>Points</strong></p> <ul class="insidebody"> <li>War and natural disasters displace populations and disrupt infrastructure and access to medical care.</li> <li>Infestations and cutaneous infections are common among refugee populations, and impetigo often is a sign of underlying scabies infestation.</li> <li>Body lice are important disease vectors inrefugee populations. </li> </ul> </itemContent> </newsItem> </itemSet></root>
Practice Points
- War and natural disasters displace populations and disrupt infrastructure and access to medical care.
- Infestations and cutaneous infections are common among refugee populations, and impetigo often is a sign of underlying scabies infestation.
- Body lice are important disease vectors inrefugee populations.
Occipital Scalp Nodule in a Newborn
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.

Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.

Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.

Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
A 4-week-old male infant was referred to dermatology for evaluation of a nodule on the occipital protuberance of 2 weeks’ duration. The patient was born at 36 weeks and 6 days’ gestation via an emergency cesarean delivery due to fetal distress. He later was found to have hypoxic-ischemic encephalopathy, pulmonary hypertension, and hypertrophic cardiomyopathy. He underwent therapeutic hypothermia protocol treatment starting at less than 6 hours after birth. At the current presentation, physical examination showed a 2.5-cm, erythematous, firm, mobile nodule on the occipital scalp with some overlying crusting and minimal surrounding erythema. No other cutaneous features or lesions were present. Initial laboratory findings were remarkable for hypercalcemia at 11 mg/dL (reference range, 8.5-10.5 mg/dL). Magnetic resonance imaging showed a faint abnormality in the subcutaneous tissue in this region without a noted connection to the underlying brain/meningeal matter. A punch biopsy was performed.

Progressively Worsening Scaly Patches and Plaques in an Infant
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
A 5-month-old male with moderately brown skin that rarely burns and tans profusely presented to the emergency department with a worsening red rash of more than 4 months’ duration. The patient had diffuse erythroderma and eczematous patches and plaques covering 95% of the total body surface area, including lichenified plaques on the arms and elbows, with no signs of infection. He initially presented for his 1-month appointment at the pediatric clinic with scaly patches and plaques on the face and trunk as well as diffuse xerosis. He was prescribed daily oatmeal baths and topical Minerin (Major Pharmaceuticals)—containing water, petrolatum, mineral oil, mineral wax, lanolin alcohol, methylchloroisothiazolinone, and methylisothiazolinone—to be applied to the whole body twice daily. At the patient’s 2-month well visit, symptoms persisted. The patient’s pediatrician increased application of Minerin to 2 to 3 times daily, and hydrocortisone cream 2.5% application 2 to 3 times daily was added.

Spring Abstract Hawaii Dermatology Seminar Compendium; Waikoloa, Hawaii; February 18-24, 2024
 unnamed.jpg
unnamed.jpg
Read More

 unnamed.jpg
unnamed.jpg
Read More

 unnamed.jpg
unnamed.jpg
Read More

Lichenoid Dermatosis on the Feet
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
An 83-year-old woman presented for evaluation of hyperkeratotic plaques on the medial and lateral aspects of the left heel (top). Physical examination also revealed onychodystrophy of the toenails on the halluces (bottom). A crusted friable plaque on the lower lip and white plaques with peripheral reticulation and erosions on the buccal mucosa also were present. The patient had a history of nummular eczema, stasis dermatitis, and hand dermatitis. She denied a history of cold sores.

Tender Dermal Nodule on the Temple
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
A 77-year-old man presented with a 1.2-cm dermal nodule on the left temple of 1 year’s duration. The lesion had become tender and darker in color. An excision was performed and submitted for histologic examination. Additional immunohistochemistry staining for Epstein-Barr virus was negative.

Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>Barker 0424</fileName> <TBEID>0C02F48C.SIG</TBEID> <TBUniqueIdentifier>NJ_0C02F48C</TBUniqueIdentifier> <newsOrJournal>Journal</newsOrJournal> <publisherName>Frontline Medical Communications Inc.</publisherName> <storyname>Botanical Briefs: Fig Phytophot</storyname> <articleType>1</articleType> <TBLocation>Copyfitting-CT</TBLocation> <QCDate/> <firstPublished>20240408T094051</firstPublished> <LastPublished>20240408T094051</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240408T094051</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Catherine Shirer Barker, MD; Thomas W. McGovern, MD</byline> <bylineText/> <bylineFull>Catherine Shirer Barker, MD; Thomas W. McGovern, MD</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>(choose one)</newsDocType> <journalDocType>(choose one)</journalDocType> <linkLabel/> <pageRange>167-169</pageRange> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:"> <name/> <rightsInfo> <copyrightHolder> <name/> </copyrightHolder> <copyrightNotice/> </rightsInfo> </provider> <abstract/> <metaDescription>Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many sp</metaDescription> <articlePDF>300906</articlePDF> <teaserImage/> <title>Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)Catherine Shirer Barker, MD; Thomas W. McGovern, MD; Dirk M. Elston, MD</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth>April</pubPubdateMonth> <pubPubdateDay/> <pubVolume>113</pubVolume> <pubNumber>4</pubNumber> <wireChannels/> <primaryCMSID/> <CMSIDs> <CMSID>2165</CMSID> </CMSIDs> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>CT</publicationCode> <pubIssueName>April 2024</pubIssueName> <pubArticleType>Audio | 2165</pubArticleType> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Cutis</journalTitle> <journalFullTitle>Cutis</journalFullTitle> <copyrightStatement>Copyright 2015 Frontline Medical Communications Inc., Parsippany, NJ, USA. All rights reserved.</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">12</term> </publications> <sections> <term canonical="true">60</term> </sections> <topics> <term canonical="true">199</term> </topics> <links> <link> <itemClass qcode="ninat:composite"/> <altRep contenttype="application/pdf">images/180026f8.pdf</altRep> <description role="drol:caption"/> <description role="drol:credit"/> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)Catherine Shirer Barker, MD; Thomas W. McGovern, MD; Dirk M. Elston, MD</title> <deck/> </itemMeta> <itemContent> <p class="abstract">Patients presenting with a linear, erythematous, blistering eruption may experience a sudden painful sunburn that seems to get worse rather than better with time. In warm climates, exposure to the common fig tree (<em>Ficus carica</em>) may be the culprit. Dermatologists should recognize fig phytophotodermatitis as a possible cause and help the patient connect their symptoms with the inciting agent as well as administer proper treatment. </p> <h3>Plant Parts and Nomenclature</h3> <p><i>Ficus</i> <i>carica </i>(common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. <i>Ficus carica </i>should not be confused with <i>Ficus benjamina</i> (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.</p> <p>The common fig tree originated in the Mediterranean and western Asia<sup>1</sup> and has been cultivated by humans since the second and third millennia <scaps>bc</scaps> for its fruit, which commonly is used to sweeten cookies, cakes, and jams.<sup>2</sup> Figs are the most commonly mentioned food plant in the Bible, with at least 56 references in the Old and New Testaments.<sup>3</sup> The “fruit” technically is a syconium—a hollow fleshy receptacle with a small opening at the apex partly closed by small scales. It can be obovoid, turbinate, or pear shaped; can be 1 to 4 inches long; and can vary in color from yellowish green to coppery, bronze, or dark purple (Figure 2).<i> <br/><br/>Ficus carica</i> is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus <i>Ficus</i> (the Latin name for a fig tree). The term <i>carica</i> likely comes from the Latin word<i> carricare</i> (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.</p> <h3>Traditional Uses</h3> <p>For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.<sup>4</sup> Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.<sup>5</sup> </p> <h3>Phototoxic Components</h3> <p>The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.<sup>6-9</sup> The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.<sup>8</sup> Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.<sup>5</sup> The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.<sup>10</sup> The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor <span class="body">α</span> and IL-1.<sup>11</sup> In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.<sup>9</sup> Humidity and sweat also increase the percutaneous absorption of psoralens.<sup>12,13</sup></p> <h3>Allergens</h3> <p>Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to <i>F benjamina </i>latex and rubber latex.<sup>6</sup> The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and <i>F benjamina </i>latex.<sup>7</sup> Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).</p> <h3>Cutaneous Manifestations</h3> <p>Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.<sup>12,14,15</sup> Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.<sup>12,13,16</sup> Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.<sup>17</sup> Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.<sup>16</sup> Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant. </p> <p>Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.<sup>12,15,16,18</sup> An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.<sup>19</sup> A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.<sup>20</sup> A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.<sup>13,14,21,22</sup> Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.<sup>22</sup> <br/><br/>The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.<sup>12,15,18</sup> Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.<sup>12</sup></p> <h3>Treatment</h3> <p>Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.<sup>15,23,24</sup> Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).</p> <h2>References</h2> <p class="reference"> 1. Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (<i>Ficus carica </i>L.) using ISSR, RAPD, and SSR markers. <i>Genetic Resources and Crop Evolution. </i>2009;56:201-209.</p> <p class="reference"> 2. Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. <i>Science. </i>1975;187:319-327.<br/><br/> 3. Young R. <i>Young’s Analytical Concordance.</i> Thomas Nelson; 1982.<br/><br/> 4. Duke JA. <i>Handbook of Medicinal Herbs.</i> CRC Press; 2002.<br/><br/> 5. Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). <i>J Invest Dermatol. </i>1959;32:509-518.<br/><br/> 6. Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between <i>Ficus benjamina</i> latex and fig fruit in patients with clinical fig allergy. <i>Clin Exp Allergy. </i>2003;33:971-977.<br/><br/> 7. Hemmer W, Focke M, Götz M, et al. Sensitization to <i>Ficus benjamina</i>: relationship to natural rubber latex allergy and identification of foods implicated in the <i>Ficus</i>-fruit syndrome. <i>Clin Exp Allergy. </i>2004;34:1251-1258.<br/><br/> 8. Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in <i>Ficus carica</i>. <i>Contact Dermatitis. </i>2010;62:343-348.<br/><br/> 9. Zaynoun ST, Aftimos BG, Abi Ali L, et al. <i>Ficus carica</i>; isolation and quantification of the photoactive components. <i>Contact Dermatitis. </i>1984;11:21-25.<br/><br/>10. Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. <i>Biochemistry. </i>1985;24:1669-1676.<br/><br/>11. Geary P. Burns related to the use of psoralens as a tanning agent. <i>Burns. </i>1996;22:636-637.<br/><br/>12. Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. <i>BMJ Case Rep. </i>2021;14:E238745.<br/><br/>13. Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. <i>J Burn Care Rehabil. </i>2003;24:229-233; discussion 228.<br/><br/>14. Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. <i>Ann Plast Surg. </i>1985;14:458-461.<br/><br/>15. Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. <i>J Pediatr. </i>2021;239:244-245.<br/><br/>16. Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. <i>Indian J Dermatol. </i>2019;64:71-73.<br/><br/>17. Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of <i>Ficus carica </i>by RP-HPLC-DAD and RP-HPLC-DAD-MS. <i>Nat Prod Commun. </i>2013;8:485-486.<br/><br/>18. Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. <i>BMJ Case Rep. </i>2020;13:<span class="cit">E233392</span>.<br/><br/>19. Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. <i>Contact Dermatitis. </i>2004;51:94-95.<br/><br/>20. Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. <i>Ulus Travma Acil Cerrahi Derg. </i>2013;19:383-384.<br/><br/>21. Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. <i>Ann Dermatol. </i>2017;29:86-90.<br/><br/>22. Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. <i>J Burn Care Res. </i>2012;33:E309-E312.<br/><br/>23. Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.<br/><br/>24. Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. <i>Adv Dermatol. </i>2008;24:105-124.</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>bio</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="disclosure">Drs. Barker and Elston are from the Medical University of South Carolina, Charleston. Dr. Barker is from the Department of Internal Medicine, and Dr. Elston is from the Department of Dermatology and Dermatologic Surgery. Dr. McGovern is from Fort Wayne Dermatology Consultants, Indiana.</p> <p class="disclosure">The authors report no conflict of interest.<br/><br/>Correspondence: Catherine Shirer Barker, MD, 96 Jonathan Lucas St, Ste 807B, MSC 623, Charleston, SC 29425 (catherinesbarker@gmail.com).</p> <p class="disclosure"><em>Cutis. </em>2024 April;113(4):167-169. doi:10.12788/cutis.0990</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>in</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="insidehead">Practice <strong>Points</strong></p> <ul class="insidebody"> <li>Exposure to the components of the common fig tree <em>(Ficus carica) </em>can induce phytophotodermatitis. </li> <li>Notable postinflammatory hyperpigmentation typically occurs in the healing stage of fig phytophotodermatitis.</li> </ul> </itemContent> </newsItem> </itemSet></root>
Practice Points
- Exposure to the components of the common fig tree (Ficus carica) can induce phytophotodermatitis.
- Notable postinflammatory hyperpigmentation typically occurs in the healing stage of fig phytophotodermatitis.
Micronutrient Deficiencies in Patients With Inflammatory Bowel Disease
In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.1 Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.2,3 Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.4 Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,5 offsetting this aversion to food can be difficult to overcome.2
Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B6 (pyridoxine), B9 (folic acid), and B12.6 Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.

Iron
A systematic review conducted from 2007 to 2012 in European patients with IBD (N=2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.7 Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.8
Pathophysiology—Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe3+ into enterocytes, where it is reduced to the transportable Fe2+.9,10 Distribution of Fe2+ ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.11 Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.12 This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.13
Cutaneous Manifestations—From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.14,15 Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency.
Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.16,17 Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).18 Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.19 An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (r=−0.768; P<.00001).20
Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.21 Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]=2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR=1.48; 95% CI, 0.32-6.82).22
Diagnosis and Monitoring—The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.23
Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.24 The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 µg/L in patients without evidence of active IBD and a ferritin level less than 100 µg/L for patients with active inflammation.25
A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 µg/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.26 In a sensitivity analysis stratifying patients by CRP level (<10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 µg/L (AUROC=0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC=0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 µg/L (AUROC=0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC=0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity >0.70) for iron deficiency in patients with Crohn disease.26
The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: r=0.66; P<.001).26 However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.27 Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration.
Treatment—Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR=2.32; P<.0001; IV: OR=3.05; P<.0001).28
Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.29 With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (P=.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.29
Zinc
A systematic review of zinc deficiency in patients with IBD identified 7 studies including 2413 patients and revealed those with Crohn disease had a higher prevalence of zinc deficiency compared with patients with ulcerative colitis (54% vs 41%).30
Pathophysiology—Zinc serves as a catalytic cofactor for enzymatic activity within proteins and immune cells.31 The homeostasis of zinc is tightly regulated within the brush border of the small intestine by zinc transporters ZIP4 and ZIP1 from the lumen of enterocytes into the bloodstream.32 Inflammation in the small intestine due to Crohn disease can result in zinc malabsorption.
Ranaldi et al33 exposed intestinal cells and zinc-depleted intestinal cells to tumor necrosis factor α media to simulate an inflammatory environment. They measured transepithelial electrical resistance as a surrogate for transmembrane permeability and found that zinc-depleted cells had a statistically significantly higher transepithelial electrical resistance percentage (60% reduction after 4 hours; P<1.10–6) when exposed to tumor necrosis factor α signaling compared with normal intestinal cells. They concluded that zinc deficiency can increase intestinal permeability in the presence of inflammation, creating a cycle of further nutrient malabsorption and inflammation exacerbating IBD symptoms.33
Cutaneous Manifestations—After absorption in the small intestine, approximately 5% of zinc resides in the skin, with the highest concentration in the stratum spinosum.34 A cell study found that keratinocytes in zinc-deficient environments had higher rates of apoptosis compared with cells in normal media. The authors proposed that this higher rate of apoptosis and the resulting inflammation could be a mechanism for developing the desquamative or eczematous scaly plaques that are common cutaneous manifestations of zinc deficiency.35
Other cutaneous findings may include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.36 The histopathology of these skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.37
Diagnosis and Monitoring—Assessing serum zinc levels is challenging, as they may decrease during states of inflammation.38 A mouse model study showed a 3.1-fold increase (P<.001) in ZIP14 expression in wild-type mice compared with an IL-6 -/- knock-down model after IL-6 exposure. The authors concluded that the upregulation of ZIP14 in the liver due to inflammatory cytokine upregulation decreases zinc availability in serum.39 Additionally, serum zinc can overestimate the level of deficiency in IBD because approximately 75% of serum zinc is bound to albumin, which decreases in the setting of inflammation.40-42
Alternatively, alkaline phosphatase (AP), a zinc-dependent metalloenzyme, may be a better evaluator of zinc status during periods of inflammation. A study in rats evaluated zinc through serum zinc levels and AP levels after a period of induced stress to mimic a short-term inflammatory state.43 The researchers found that total body stores of zinc were unaffected throughout the experiment; only serum zinc declined throughout the experiment duration while AP did not. Because approximately 75% of serum zinc is bound to serum albumin,42 the researchers concluded the induced inflammatory state depleted serum albumin and redistributed zinc to the liver, causing the observed serum zinc changes, while total body zinc levels and AP were largely unaffected in comparison.43 Comorbid conditions such as liver or bone disease can increase AP levels, which limits the utility of AP as a surrogate for zinc in patients with comorbidities.44 However, even in the context of active IBD, serum zinc still is currently considered the best biomarker to evaluate zinc status.45
Treatment—The recommended dose for zinc supplementation is 20 to 40 mg daily with higher doses (>50 mg/d) for patients with malabsorptive syndromes such as IBD.46 It can be administered orally or parenterally. Although rare, zinc replacement therapy may be associated with diarrhea, nausea, vomiting, mild headaches, and fatigue.46 Additional considerations should be taken when repleting other micronutrients with zinc, as calcium and folate can inhibit zinc reabsorption, while zinc itself can inhibit iron and copper reabsorption.47
Vitamin D and Calcium
Low vitamin D levels (<50 nmol/L) and hypocalcemia (<8.8 mg/dL) are common in patients with IBD.48,49
Pathophysiology—Vitamin D levels are maintained via 2 mechanisms. The first mechanism is through the skin, as keratinocytes produce 7-dehydrocholesterol after exposure to UV light, which is converted into previtamin D3 and then thermally isomerizes into vitamin D3. This vitamin D3 is then transported to the liver on vitamin D–binding protein.50 The second mechanism is through oral vitamin D3 that is absorbed through vitamin D receptors in intestinal epithelium and transported to the liver, where it is hydroxylated into 25-hydroxyvitamin D (25[OH]D), then to the kidneys for hydroxylation to 1,25(OH)2D for redistribution throughout the body.50 This activated form of vitamin D regulates calcium absorption in the intestine, and optimal vitamin D levels are necessary to absorb calcium efficiently.51 Inflammation from IBD within the small intestine can downregulate vitamin D receptors, causing malabsorption and decreased serum vitamin D.52
Vitamin D signaling also is vital to maintaining the tight junctions and adherens junctions of the intestinal epithelium. Weakening the permeability of the epithelium further exacerbates malabsorption and subsequent vitamin D deficiency.52 A meta-analysis of 27 studies including 8316 patients with IBD showed low vitamin D levels were associated with increased odds of disease activity (OR=1.53; 95% CI, 1.32-1.77), mucosal inflammation (OR=1.25; 95% CI, 1.06-1.47), and future clinical relapse (OR=1.23; 95% CI, 1.03-1.47) in patients with Crohn disease. The authors concluded that low levels of vitamin D could be used as a potential biomarker of inflammatory status in Crohn disease.53
Vitamin D and calcium are further implicated in maintaining skeletal health,47 while vitamin D specifically helps maintain intestinal homeostasis54 and immune system modulation in the skin.55
Cutaneous Manifestations—Vitamin D is thought to play crucial roles in skin differentiation and proliferation, cutaneous innate immunity, hair follicle cycling, photoprotection, and wound healing.56 Vitamin D deficiency has been observed in a large range of cutaneous diseases including skin cancer, psoriasis, vitiligo, bullous pemphigoid, atopic dermatitis, and various types of alopecia.56-59 It is unclear whether vitamin D deficiency facilitates these disease processes or is merely the consequence of a disrupted cutaneous surface with the inability to complete the first step in vitamin D processing. A 2014 meta-analysis of 290 prospective cohort studies and 172 randomized trials concluded that 25(OH)D deficiency was associated with ill health and did not find causal evidence for any specific disease, dermatologic or otherwise.60 Calcium deficiency may cause epidermal changes including dry skin, coarse hair, and brittle nails.61
Diagnosis and Monitoring—The ECCO guidelines recommend obtaining serum 25(OH)D levels every 3 months in patients with IBD.62 Levels less than 75 nmol/L are considered deficient, and a value less than 30 nmol/L increases the risk for osteomalacia and nutritional rickets, constituting severe vitamin D deficiency.63-65
An observational study of 325 patients with IBD showed a statistically significant negative correlation between serum vitamin D and fecal calprotectin (r=−0.19; P<.001), a stool-based marker for gut inflammation, supporting vitamin D as a potential biomarker in IBD.66
Evaluation of calcium can be done through serum levels in patients with IBD.67 Patients with IBD are at risk for hypoalbuminemia; therefore, consideration should be taken to ensure calcium levels are corrected, as approximately 50% of calcium is bound to albumin or other ions in the body,68 which can be done by adjusting the calcium concentration by 0.02 mmol/L for every 1 g/L of albumin above or below 40 g/L. In the most critically ill patients, a direct ionized calcium blood level should be used instead because the previously mentioned correction calculations are inaccurate when albumin is critically low.69
Treatment—The ECCO guidelines recommend calcium and vitamin D repletion of 500 to 1000 mg and 800 to 1000 U, respectively, in patients with IBD on systemic corticosteroids to prevent the negative effects of bone loss.62 Calcium repletion in patients with IBD who are not on systemic steroids are the same as for the general population.65
Vitamin D repletion also may help decrease IBD activity. In a prospective study, 10,000 IU/d of vitamin D in 10 patients with IBD—adjusted over 12 weeks to a target of 100 to 125 nmol/L of serum 25(OH)D—showed a significant reduction in clinical Crohn activity (P=.019) over the study period.70 In contrast, 2000 IU/d for 3 months in an RCT of 27 patients with Crohn disease found significantly lower CRP (P=.019) and significantly higher self-reported quality of life (P=.037) but nonsignificant decreases in Crohn activity (P=.082) in patients with 25(OH)D levels of 75 nmol/L or higher compared with those with 25(OH)D levels less than 75 nmol/L.71
These discrepancies illustrate the need for expanded clinical trials to elucidate the optimal vitamin D dosing for patients with IBD. Ultimately, assessing vitamin D and calcium status and considering repletion in patients with IBD, especially those with comorbid dermatologic diseases such as poor wound healing, psoriasis, or atopic dermatitis, is important.
Vitamin B6 (Pyridoxine)
Pathophysiology—Pyridoxine is an important coenzyme for many functions including amino acid transamination, fatty acid metabolism, and conversion of tryptophan to niacin. It is absorbed in the jejunum and ileum and subsequently transported to the liver for rephosphorylation and release into its active form.36 An observational study assessing the nutritional status of patients with IBD found that only 5.7% of 105 patients with food records had inadequate dietary intake of pyridoxine, but 29% of all patients with IBD had subnormal pyridoxine levels.72 Additionally, they found no significant difference in the prevalence of subnormal pyridoxine levels in patients with active IBD vs IBD in remission. The authors suggested that the subnormal pyridoxine levels in patients with IBD likely were multifactorial and resulted from malabsorption due to active disease, inflammation, and inadequate intake.72
Cutaneous Manifestations—Cutaneous findings associated with pyridoxine deficiency include periorificial and perineal dermatitis,73 angular stomatitis, and cheilitis with associated burning, redness, and tongue edema.36 Additionally, pyridoxine is involved in the conversion of tryptophan to niacin, and its deficiency may manifest with pellagralike findings.74
Because pyridoxine is critical to protein metabolism, its deficiency may disrupt key cellular structures that rely on protein concentrations to maintain structural integrity. One such structure in the skin that heavily relies on protein concentrations is the ground substance of the extracellular matrix—the amorphous gelatinous spaces that occupy the areas between the extracellular matrix, which consists of cross-linked glycosaminoglycans and proteins.75 Without protein, ground substance increases in viscosity and can disrupt the epidermal barrier, leading to increased transepidermal water loss and ultimately inflammation.76 Although this theory has yet to be validated fully, this is a potential mechanistic explanation for the inflammation in dermal papillae that leads to dermatitis observed in pyridoxine deficiency.
Diagnosis and Monitoring—Direct biomarkers of pyridoxine status are in serum, plasma, erythrocytes, and urine, with the most common measurement in plasma as pyridoxal 5′-phosphate (PLP).77 Plasma PLP concentrations lower than 20 nmol/L are suggestive of deficiency.78 Plasma PLP has shown inverse relationships with acute phase inflammatory markers CRP79 and AP,78 thereby raising concerns for its validity to assess pyridoxine status in patients with symptomatic IBD.80
Alternative evaluations of pyridoxine include tryptophan and methionine loading tests,36 which are measured via urinary excretion and require normal kidney function to be accurate. They should be considered in IBD if necessary, but routine testing, even in patients with symptomatic IBD, is not recommended in the ECCO guidelines. Additional considerations should be taken in patients with altered nutrient requirements such as those who have undergone bowel resection due to highly active disease or those who receive parenteral nutritional supplementation.81
Treatment—Recommendations for oral pyridoxine supplementation range from 25 to 600 mg daily,82 with symptoms typically improving on 100 mg daily.36 Pyridoxine supplementation may have additional benefits for patients with IBD and potentially modulate disease severity. An IL-10 knockout mouse supplemented with pyridoxine had an approximately 60% reduction (P<.05) in inflammation compared to mice deficient in pyridoxine.83 The authors suggest that PLP-dependent enzymes can inhibit further proinflammatory signaling and T-cell migration that can exacerbate IBD. Ultimately, more data is needed before determining the efficacy of pyridoxine supplementation for active IBD.
Vitamin B12 and Vitamin B9 (Folic Acid)
Pathophysiology—Vitamin B12 is reabsorbed in the terminal ileum, the distal portion of the small intestine. The American Gastroenterological Association recommends that patients with a history of extensive ileal disease or prior ileal surgery, which is the case for many patients with Crohn disease, be monitored for vitamin B12 deficiency.23 Monitoring and rapid supplementation of vitamin B12 can prevent pernicious anemia and irreversible neurologic damage that may result from deficiency.84
Folic acid is primarily absorbed in the duodenum and jejunum of the small intestine. A meta-analysis performed in 2017 assessed studies observing folic acid and vitamin B12 levels in 1086 patients with IBD compared with 1484 healthy controls and found an average difference in serum folate concentration of 0.46 nmol/L (P<.001).84 Interestingly, this study did not find a significant difference in serum vitamin B12 levels between patients with IBD and healthy controls, highlighting the mechanism of vitamin B12 deficiency in IBD because only patients with terminal ileal involvement are at risk for malabsorption and subsequent deficiency.
Cutaneous Manifestations—Both vitamin B12 and folic acid deficiency can manifest as cheilitis, glossitis, and/or generalized hyperpigmentation that is accentuated in the flexural areas, palms, soles, and oral cavity.85,86 Systemic symptoms of patients with vitamin B12 and folic acid deficiency include megaloblastic anemia, pallor, and fatigue. A potential mechanism for the hyperpigmentation observed from vitamin B12 deficiency came from an electron microscope study that showed an increased concentration of melanosomes in a patient with deficiency.87
Diagnosis and Monitoring—In patients with suspected vitamin B12 and/or folic acid deficiency, initial evaluation should include a CBC with peripheral smear and serum vitamin B12 and folate levels. In cases for which the diagnosis still is unclear after initial testing,
Treatment—According to the Centers for Disease Control and Prevention, supplementation of vitamin B12 can be done orally with 1000 µg daily in patients with deficiency. In patients with active IBD, oral reabsorption of vitamin B12 can be less effective, making subcutaneous or intramuscular administration (1000 µg/wk for 8 weeks, then monthly for life) better options.89
Patients with IBD managed with methotrexate should be screened carefully for folate deficiency. Methotrexate is a folate analog that sometimes is used for the treatment of IBD. Reversible competitive inhibition of dihydrofolate reductase can precipitate a systemic folic acid decrease.91 Typically, oral folic acid (1 to 5 mg/d) is sufficient to treat folate deficiency, with the ESPEN recommending 5 mg once weekly 24 to 72 hours after methotrexate treatment or 1 mg daily for 5 days per week in patients with IBD.1 Alternative formulations—IV, subcutaneous, or intramuscular—are available for patients who cannot tolerate oral intake.92
Final Thoughts
Dermatologists can be the first to observe the cutaneous manifestations of micronutrient deficiencies. Although the symptoms of each micronutrient deficiency discussed may overlap, attention to small clinical clues in patients with IBD can improve patient outcomes and quality of life. For example, koilonychia with glossitis and xerosis likely is due to iron deficiency, while zinc deficiency should be suspected in patients with scaly eczematous plaques in skin folds. A high level of suspicion for micronutrient deficiencies in patients with IBD should be followed by a complete patient history, review of systems, and thorough clinical examination. A thorough laboratory evaluation can pinpoint nutritional deficiencies in patients with IBD, keeping in mind that specific biomarkers such as ferritin and serum zinc also act as acute phase reactants and should be interpreted in this context. Co-management with gastroenterologists should be a priority in patients with IBD, as gaining control of inflammatory disease is crucial for the prevention of recurrent vitamin and micronutrient deficiencies in addition to long-term health in this population.
- Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin Nutr. 2023;42:352-379. doi:10.1016/j.clnu.2022.12.004
- Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammator y bowel disease. J Hum Nutr Diet. 2011;24:313-326. doi:10.1111/j.1365-277X.2011.01171.x
- Mentella MC, Scaldaferri F, Pizzoferrato M, et al. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020;12:944. doi:10.3390/nu12040944
- Bonsack O, Caron B, Baumann C, et al. Food avoidance and fasting in patients with inflammatory bowel disease: experience from the Nancy IBD nutrition clinic. United European Gastroenterol J. 2023;11:361-370. doi:10.1002/ueg2.1238521
- Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;13:1067. doi:10.3390/nu13041067
- Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. Clin Gastroenterol Hepatol. 2020;18:2645-2649.e4. doi:10.1016/j.cgh.2020.06.039
- Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. doi:10.1097/01.MIB.0000442728.74340.fd
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. doi:10.1038/nrgastro.2010.151
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls [Internet]. Updated April 17, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448204/
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. doi:10.1136/gut.2010.214312
- Przybyszewska J, Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol. 2014;9:208-213. doi:10.5114/pg.2014.45102
- Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175-178. doi:10.3324/haematol.2009.017046
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. doi:10.4291/wjgp.v6.i3.62
- Moiz B. Spoon nails: still seen in today’s world. Clin Case Rep. 2018;6:547-548. doi:10.1002/ccr3.1404
- St Pierre SA, Vercellotti GM, Donovan JC, et al. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070-1076. doi:10.1016/j.jaad.2009.05.054
- Chiang CP, Yu-Fong Chang J, Wang YP, et al. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in atrophic glossitis patients with or without microcytosis. J Formos Med Assoc. 2019;118:1401-1407. doi:10.1016/j.jfma.2019.06.004
- Chiang CP, Chang JY, Wang YP, et al. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J Formos Med Assoc. 2020;119:774-780. doi:10.1016/j.jfma.2019.04.015
- Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi:10.1111/jdv.13610
- Guo HF, Tsai CL, Terajima M, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding. Nat Commun. 2018;9:512. doi:10.1038/s41467-018-02859-z
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088-1092. doi:10.1126/science.1157121
- Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-477.e16. doi:10.1016/j.jaad.2018.07.013
- Hashash JG, Elkins J, Lewis JD, et al. AGA Clinical Practice Update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review [published online January 23, 2024]. Gastroenterology. doi:10.1053/j.gastro.2023.11.303
- Choudhuri S, Chowdhury IH, Saha A, et al. Acute monocyte pro- inflammatory response predicts higher positive to negative acute phase reactants ratio and severe hemostatic derangement in dengue fever. Cytokine. 2021;146:155644. doi:10.1016/j.cyto.2021.155644
- Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohn’s Colitis. 2015;9:211-222. doi:10.1093/ecco-jcc/jju009
- Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:1087-1095. doi:10.1111/apt.15739
- Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(suppl 6):1606S-1614S. doi:10.3945/ajcn.117.155887
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. doi:10.1371/journal.pone.0117383
- Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e8532. doi:10.1053/j.gastro.2011.06.005
- Zupo R, Sila A, Castellana F, et al. Prevalence of zinc deficiency in inflammatory bowel disease: a systematic review and meta-analysis. Nutrients. 2022;14:4052. doi:10.3390/nu14194052
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals. 2022;35:187-213. doi:10.1007/s10534-022-00373-w
- Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12:762. doi:10.3390/nu12030762
- Ranaldi G, Ferruzza S, Canali R, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967-976. doi:10.1016/j.jnutbio.2012.06.020
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016;611:113-119. doi:10.1016/j.abb.2016.06.003
- Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol. 2006;84:28-37. doi:10.1111/j.1440-1711.2005.01391.x
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685. doi:10.1016/j.clindermatol.2010.03.029
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi:10.3390/nu9060624
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843-6848. doi:10.1073/pnas.0502257102
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. Gut. 2006;55:426-431. doi:10.1136/gut.2005.069476
- Morisaku M, Ito K, Ogiso A, et al. Correlation between serum albumin and serum zinc in malignant lymphoma. Fujita Med J. 2022;8:59-64. doi:10.20407/fmj.2021-006
- Falchuk KH. Effect of acute disease and ACTH on serum zinc proteins. N Engl J Med. 1977:296:1129-1134.
- Naber TH, Baadenhuysen H, Jansen JB, et al. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin Chim Acta. 1996;249:109-127. doi:10.1016/0009-8981(96)06281-x
- Lowe D, Sanvictores T, Zubair M, et al. Alkaline phosphatase. StatPearls [Internet]. Updated October 29, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459201/
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 suppl 1:19-29. doi:10.1159/000348261
- Maxfield L, Shukla S, Crane JS. Zinc deficiency. StatPearls [Internet]. Updated June 28, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493231/
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808. doi:10.1016/j.gtc.2017.08.011
- Caviezel D, Maissen S, Niess JH, et al. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm Intest Dis. 2018;2:200-210. doi:10.1159/000489010
- Jasielska M, Grzybowska-Chlebowczyk U. Hypocalcemia and vitamin D deficiency in children with inflammatory bowel diseases and lactose intolerance. Nutrients. 2021;13:2583. doi:10.3390/nu13082583
- Vernia F, Valvano M, Longo S, et al. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients. 2022;14:269. doi:10.3390/nu14020269
- Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. doi:10.1007/s11926-008-0020-y
- Domazetovic V, Iantomasi T, Bonanomi AG, et al. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int J Colorectal Dis. 2020;35:1231-1242. doi:10.1007/s00384-020-03576-0
- Gubatan J, Chou ND, Nielsen OH, et al. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146-1158. doi:10.1111/apt.15506
- Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi:10.1016/j.jsbmb.2020.105663
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. doi:10.1126/science.1123933
- Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793-804. doi:10.1016/j.jare.2014.01.011
- Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397-409.
- Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43:529-535.
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404-412.
- Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. doi:10.1016/S2213-8587(13)70165-7
- Schafer AL, Shoback DM. Hypocalcemia: diagnosis and treatment. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. Updated January 3, 2016. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK279022/
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649-670. doi:10.1093/ecco-jcc/jjx008
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415. doi:10.1210/jc.2015-2175
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US); 2011.
- Yeaman F, Nguyen A, Abasszade J, et al. Assessing vitamin D as a biomarker in inflammatory bowel disease. JGH Open. 2023;7:953-958. doi:10.1002/jgh3.13010
- Vernia P, Loizos P, Di Giuseppantonio I, et al S. Dietary calcium intake in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:312-317. doi:10.1016/j.crohns.2013.09.008
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298-1302. doi:10.1136/bmj.39582.589433.BE
- Kenny CM, Murphy CE, Boyce DS, et al. Things we do for no reason™: calculating a “corrected calcium” level. J Hosp Med. 2021;16:499-501. doi:10.12788/jhm.3619
- Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr. 2018;37:1375-1382. doi:10.1016/j.clnu.2017.06.011
- Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294-302. doi:10.1177/2050640615572176
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. doi:10.1177/0148607107031004311
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274. doi:10.1016/s0190-9622(86)70301-0
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739. doi:10.3949/ccjm.83a.15061
- Elgharably N, Al Abadie M, Al Abadie M, et al. Vitamin B group levels and supplementations in dermatology. Dermatol Reports. 2022;15:9511. doi:10.4081/dr.2022.9511
- Hołubiec P, Leon´czyk M, Staszewski F, et al. Pathophysiology and clinical management of pellagra—a review. Folia Med Cracov. 2021;61:125-137. doi:10.24425/fmc.2021.138956
- Ink SL, Henderson LM. Vitamin B6 metabolism. Annu Rev Nutr. 1984;4:455-470. doi:10.1146/annurev.nu.04.070184.002323
- Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 deficiency. StatPearls [Internet]. Updated August 8, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470579/.
- Vasilaki AT, McMillan DC, Kinsella J, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr. 2008;88:140-146. doi:10.1093/ajcn/88.1.140
- Chiang EP, Bagley PJ, Selhub J, et al. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283-287. doi:10.1016/s0002-9343(02)01528-0
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. doi:10.1093/ecco-jcc/jjy113
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138-2143. doi:10.1016/j.jnutbio.2013.08.005
- Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. doi:10.3390/nu9040382
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33. doi:10.1007/s40257-014-0107-3
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503. doi:10.1002/ncp.10321
- Mori K, Ando I, Kukita A. Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J Dermatol. 2001;28:282-285. doi:10.1111/j.1346-8138.2001.tb00134.x
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011;94:666S-672S. doi:10.3945/ajcn.110.009613
- NIH Office of Dietary Supplements. Vitamin B12: fact sheet for health professionals. Updated February 27, 2024. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
- NIH Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 20, 2023. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.
- Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123-127. doi:10.1016/j.dld.2011.09.015
- Khan KM, Jialal I. Folic acid deficiency. StatPearls [Internet]. Updated June 26, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535377/
In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.1 Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.2,3 Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.4 Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,5 offsetting this aversion to food can be difficult to overcome.2
Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B6 (pyridoxine), B9 (folic acid), and B12.6 Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.

Iron
A systematic review conducted from 2007 to 2012 in European patients with IBD (N=2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.7 Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.8
Pathophysiology—Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe3+ into enterocytes, where it is reduced to the transportable Fe2+.9,10 Distribution of Fe2+ ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.11 Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.12 This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.13
Cutaneous Manifestations—From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.14,15 Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency.
Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.16,17 Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).18 Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.19 An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (r=−0.768; P<.00001).20
Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.21 Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]=2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR=1.48; 95% CI, 0.32-6.82).22
Diagnosis and Monitoring—The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.23
Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.24 The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 µg/L in patients without evidence of active IBD and a ferritin level less than 100 µg/L for patients with active inflammation.25
A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 µg/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.26 In a sensitivity analysis stratifying patients by CRP level (<10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 µg/L (AUROC=0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC=0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 µg/L (AUROC=0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC=0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity >0.70) for iron deficiency in patients with Crohn disease.26
The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: r=0.66; P<.001).26 However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.27 Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration.
Treatment—Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR=2.32; P<.0001; IV: OR=3.05; P<.0001).28
Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.29 With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (P=.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.29
Zinc
A systematic review of zinc deficiency in patients with IBD identified 7 studies including 2413 patients and revealed those with Crohn disease had a higher prevalence of zinc deficiency compared with patients with ulcerative colitis (54% vs 41%).30
Pathophysiology—Zinc serves as a catalytic cofactor for enzymatic activity within proteins and immune cells.31 The homeostasis of zinc is tightly regulated within the brush border of the small intestine by zinc transporters ZIP4 and ZIP1 from the lumen of enterocytes into the bloodstream.32 Inflammation in the small intestine due to Crohn disease can result in zinc malabsorption.
Ranaldi et al33 exposed intestinal cells and zinc-depleted intestinal cells to tumor necrosis factor α media to simulate an inflammatory environment. They measured transepithelial electrical resistance as a surrogate for transmembrane permeability and found that zinc-depleted cells had a statistically significantly higher transepithelial electrical resistance percentage (60% reduction after 4 hours; P<1.10–6) when exposed to tumor necrosis factor α signaling compared with normal intestinal cells. They concluded that zinc deficiency can increase intestinal permeability in the presence of inflammation, creating a cycle of further nutrient malabsorption and inflammation exacerbating IBD symptoms.33
Cutaneous Manifestations—After absorption in the small intestine, approximately 5% of zinc resides in the skin, with the highest concentration in the stratum spinosum.34 A cell study found that keratinocytes in zinc-deficient environments had higher rates of apoptosis compared with cells in normal media. The authors proposed that this higher rate of apoptosis and the resulting inflammation could be a mechanism for developing the desquamative or eczematous scaly plaques that are common cutaneous manifestations of zinc deficiency.35
Other cutaneous findings may include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.36 The histopathology of these skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.37
Diagnosis and Monitoring—Assessing serum zinc levels is challenging, as they may decrease during states of inflammation.38 A mouse model study showed a 3.1-fold increase (P<.001) in ZIP14 expression in wild-type mice compared with an IL-6 -/- knock-down model after IL-6 exposure. The authors concluded that the upregulation of ZIP14 in the liver due to inflammatory cytokine upregulation decreases zinc availability in serum.39 Additionally, serum zinc can overestimate the level of deficiency in IBD because approximately 75% of serum zinc is bound to albumin, which decreases in the setting of inflammation.40-42
Alternatively, alkaline phosphatase (AP), a zinc-dependent metalloenzyme, may be a better evaluator of zinc status during periods of inflammation. A study in rats evaluated zinc through serum zinc levels and AP levels after a period of induced stress to mimic a short-term inflammatory state.43 The researchers found that total body stores of zinc were unaffected throughout the experiment; only serum zinc declined throughout the experiment duration while AP did not. Because approximately 75% of serum zinc is bound to serum albumin,42 the researchers concluded the induced inflammatory state depleted serum albumin and redistributed zinc to the liver, causing the observed serum zinc changes, while total body zinc levels and AP were largely unaffected in comparison.43 Comorbid conditions such as liver or bone disease can increase AP levels, which limits the utility of AP as a surrogate for zinc in patients with comorbidities.44 However, even in the context of active IBD, serum zinc still is currently considered the best biomarker to evaluate zinc status.45
Treatment—The recommended dose for zinc supplementation is 20 to 40 mg daily with higher doses (>50 mg/d) for patients with malabsorptive syndromes such as IBD.46 It can be administered orally or parenterally. Although rare, zinc replacement therapy may be associated with diarrhea, nausea, vomiting, mild headaches, and fatigue.46 Additional considerations should be taken when repleting other micronutrients with zinc, as calcium and folate can inhibit zinc reabsorption, while zinc itself can inhibit iron and copper reabsorption.47
Vitamin D and Calcium
Low vitamin D levels (<50 nmol/L) and hypocalcemia (<8.8 mg/dL) are common in patients with IBD.48,49
Pathophysiology—Vitamin D levels are maintained via 2 mechanisms. The first mechanism is through the skin, as keratinocytes produce 7-dehydrocholesterol after exposure to UV light, which is converted into previtamin D3 and then thermally isomerizes into vitamin D3. This vitamin D3 is then transported to the liver on vitamin D–binding protein.50 The second mechanism is through oral vitamin D3 that is absorbed through vitamin D receptors in intestinal epithelium and transported to the liver, where it is hydroxylated into 25-hydroxyvitamin D (25[OH]D), then to the kidneys for hydroxylation to 1,25(OH)2D for redistribution throughout the body.50 This activated form of vitamin D regulates calcium absorption in the intestine, and optimal vitamin D levels are necessary to absorb calcium efficiently.51 Inflammation from IBD within the small intestine can downregulate vitamin D receptors, causing malabsorption and decreased serum vitamin D.52
Vitamin D signaling also is vital to maintaining the tight junctions and adherens junctions of the intestinal epithelium. Weakening the permeability of the epithelium further exacerbates malabsorption and subsequent vitamin D deficiency.52 A meta-analysis of 27 studies including 8316 patients with IBD showed low vitamin D levels were associated with increased odds of disease activity (OR=1.53; 95% CI, 1.32-1.77), mucosal inflammation (OR=1.25; 95% CI, 1.06-1.47), and future clinical relapse (OR=1.23; 95% CI, 1.03-1.47) in patients with Crohn disease. The authors concluded that low levels of vitamin D could be used as a potential biomarker of inflammatory status in Crohn disease.53
Vitamin D and calcium are further implicated in maintaining skeletal health,47 while vitamin D specifically helps maintain intestinal homeostasis54 and immune system modulation in the skin.55
Cutaneous Manifestations—Vitamin D is thought to play crucial roles in skin differentiation and proliferation, cutaneous innate immunity, hair follicle cycling, photoprotection, and wound healing.56 Vitamin D deficiency has been observed in a large range of cutaneous diseases including skin cancer, psoriasis, vitiligo, bullous pemphigoid, atopic dermatitis, and various types of alopecia.56-59 It is unclear whether vitamin D deficiency facilitates these disease processes or is merely the consequence of a disrupted cutaneous surface with the inability to complete the first step in vitamin D processing. A 2014 meta-analysis of 290 prospective cohort studies and 172 randomized trials concluded that 25(OH)D deficiency was associated with ill health and did not find causal evidence for any specific disease, dermatologic or otherwise.60 Calcium deficiency may cause epidermal changes including dry skin, coarse hair, and brittle nails.61
Diagnosis and Monitoring—The ECCO guidelines recommend obtaining serum 25(OH)D levels every 3 months in patients with IBD.62 Levels less than 75 nmol/L are considered deficient, and a value less than 30 nmol/L increases the risk for osteomalacia and nutritional rickets, constituting severe vitamin D deficiency.63-65
An observational study of 325 patients with IBD showed a statistically significant negative correlation between serum vitamin D and fecal calprotectin (r=−0.19; P<.001), a stool-based marker for gut inflammation, supporting vitamin D as a potential biomarker in IBD.66
Evaluation of calcium can be done through serum levels in patients with IBD.67 Patients with IBD are at risk for hypoalbuminemia; therefore, consideration should be taken to ensure calcium levels are corrected, as approximately 50% of calcium is bound to albumin or other ions in the body,68 which can be done by adjusting the calcium concentration by 0.02 mmol/L for every 1 g/L of albumin above or below 40 g/L. In the most critically ill patients, a direct ionized calcium blood level should be used instead because the previously mentioned correction calculations are inaccurate when albumin is critically low.69
Treatment—The ECCO guidelines recommend calcium and vitamin D repletion of 500 to 1000 mg and 800 to 1000 U, respectively, in patients with IBD on systemic corticosteroids to prevent the negative effects of bone loss.62 Calcium repletion in patients with IBD who are not on systemic steroids are the same as for the general population.65
Vitamin D repletion also may help decrease IBD activity. In a prospective study, 10,000 IU/d of vitamin D in 10 patients with IBD—adjusted over 12 weeks to a target of 100 to 125 nmol/L of serum 25(OH)D—showed a significant reduction in clinical Crohn activity (P=.019) over the study period.70 In contrast, 2000 IU/d for 3 months in an RCT of 27 patients with Crohn disease found significantly lower CRP (P=.019) and significantly higher self-reported quality of life (P=.037) but nonsignificant decreases in Crohn activity (P=.082) in patients with 25(OH)D levels of 75 nmol/L or higher compared with those with 25(OH)D levels less than 75 nmol/L.71
These discrepancies illustrate the need for expanded clinical trials to elucidate the optimal vitamin D dosing for patients with IBD. Ultimately, assessing vitamin D and calcium status and considering repletion in patients with IBD, especially those with comorbid dermatologic diseases such as poor wound healing, psoriasis, or atopic dermatitis, is important.
Vitamin B6 (Pyridoxine)
Pathophysiology—Pyridoxine is an important coenzyme for many functions including amino acid transamination, fatty acid metabolism, and conversion of tryptophan to niacin. It is absorbed in the jejunum and ileum and subsequently transported to the liver for rephosphorylation and release into its active form.36 An observational study assessing the nutritional status of patients with IBD found that only 5.7% of 105 patients with food records had inadequate dietary intake of pyridoxine, but 29% of all patients with IBD had subnormal pyridoxine levels.72 Additionally, they found no significant difference in the prevalence of subnormal pyridoxine levels in patients with active IBD vs IBD in remission. The authors suggested that the subnormal pyridoxine levels in patients with IBD likely were multifactorial and resulted from malabsorption due to active disease, inflammation, and inadequate intake.72
Cutaneous Manifestations—Cutaneous findings associated with pyridoxine deficiency include periorificial and perineal dermatitis,73 angular stomatitis, and cheilitis with associated burning, redness, and tongue edema.36 Additionally, pyridoxine is involved in the conversion of tryptophan to niacin, and its deficiency may manifest with pellagralike findings.74
Because pyridoxine is critical to protein metabolism, its deficiency may disrupt key cellular structures that rely on protein concentrations to maintain structural integrity. One such structure in the skin that heavily relies on protein concentrations is the ground substance of the extracellular matrix—the amorphous gelatinous spaces that occupy the areas between the extracellular matrix, which consists of cross-linked glycosaminoglycans and proteins.75 Without protein, ground substance increases in viscosity and can disrupt the epidermal barrier, leading to increased transepidermal water loss and ultimately inflammation.76 Although this theory has yet to be validated fully, this is a potential mechanistic explanation for the inflammation in dermal papillae that leads to dermatitis observed in pyridoxine deficiency.
Diagnosis and Monitoring—Direct biomarkers of pyridoxine status are in serum, plasma, erythrocytes, and urine, with the most common measurement in plasma as pyridoxal 5′-phosphate (PLP).77 Plasma PLP concentrations lower than 20 nmol/L are suggestive of deficiency.78 Plasma PLP has shown inverse relationships with acute phase inflammatory markers CRP79 and AP,78 thereby raising concerns for its validity to assess pyridoxine status in patients with symptomatic IBD.80
Alternative evaluations of pyridoxine include tryptophan and methionine loading tests,36 which are measured via urinary excretion and require normal kidney function to be accurate. They should be considered in IBD if necessary, but routine testing, even in patients with symptomatic IBD, is not recommended in the ECCO guidelines. Additional considerations should be taken in patients with altered nutrient requirements such as those who have undergone bowel resection due to highly active disease or those who receive parenteral nutritional supplementation.81
Treatment—Recommendations for oral pyridoxine supplementation range from 25 to 600 mg daily,82 with symptoms typically improving on 100 mg daily.36 Pyridoxine supplementation may have additional benefits for patients with IBD and potentially modulate disease severity. An IL-10 knockout mouse supplemented with pyridoxine had an approximately 60% reduction (P<.05) in inflammation compared to mice deficient in pyridoxine.83 The authors suggest that PLP-dependent enzymes can inhibit further proinflammatory signaling and T-cell migration that can exacerbate IBD. Ultimately, more data is needed before determining the efficacy of pyridoxine supplementation for active IBD.
Vitamin B12 and Vitamin B9 (Folic Acid)
Pathophysiology—Vitamin B12 is reabsorbed in the terminal ileum, the distal portion of the small intestine. The American Gastroenterological Association recommends that patients with a history of extensive ileal disease or prior ileal surgery, which is the case for many patients with Crohn disease, be monitored for vitamin B12 deficiency.23 Monitoring and rapid supplementation of vitamin B12 can prevent pernicious anemia and irreversible neurologic damage that may result from deficiency.84
Folic acid is primarily absorbed in the duodenum and jejunum of the small intestine. A meta-analysis performed in 2017 assessed studies observing folic acid and vitamin B12 levels in 1086 patients with IBD compared with 1484 healthy controls and found an average difference in serum folate concentration of 0.46 nmol/L (P<.001).84 Interestingly, this study did not find a significant difference in serum vitamin B12 levels between patients with IBD and healthy controls, highlighting the mechanism of vitamin B12 deficiency in IBD because only patients with terminal ileal involvement are at risk for malabsorption and subsequent deficiency.
Cutaneous Manifestations—Both vitamin B12 and folic acid deficiency can manifest as cheilitis, glossitis, and/or generalized hyperpigmentation that is accentuated in the flexural areas, palms, soles, and oral cavity.85,86 Systemic symptoms of patients with vitamin B12 and folic acid deficiency include megaloblastic anemia, pallor, and fatigue. A potential mechanism for the hyperpigmentation observed from vitamin B12 deficiency came from an electron microscope study that showed an increased concentration of melanosomes in a patient with deficiency.87
Diagnosis and Monitoring—In patients with suspected vitamin B12 and/or folic acid deficiency, initial evaluation should include a CBC with peripheral smear and serum vitamin B12 and folate levels. In cases for which the diagnosis still is unclear after initial testing,
Treatment—According to the Centers for Disease Control and Prevention, supplementation of vitamin B12 can be done orally with 1000 µg daily in patients with deficiency. In patients with active IBD, oral reabsorption of vitamin B12 can be less effective, making subcutaneous or intramuscular administration (1000 µg/wk for 8 weeks, then monthly for life) better options.89
Patients with IBD managed with methotrexate should be screened carefully for folate deficiency. Methotrexate is a folate analog that sometimes is used for the treatment of IBD. Reversible competitive inhibition of dihydrofolate reductase can precipitate a systemic folic acid decrease.91 Typically, oral folic acid (1 to 5 mg/d) is sufficient to treat folate deficiency, with the ESPEN recommending 5 mg once weekly 24 to 72 hours after methotrexate treatment or 1 mg daily for 5 days per week in patients with IBD.1 Alternative formulations—IV, subcutaneous, or intramuscular—are available for patients who cannot tolerate oral intake.92
Final Thoughts
Dermatologists can be the first to observe the cutaneous manifestations of micronutrient deficiencies. Although the symptoms of each micronutrient deficiency discussed may overlap, attention to small clinical clues in patients with IBD can improve patient outcomes and quality of life. For example, koilonychia with glossitis and xerosis likely is due to iron deficiency, while zinc deficiency should be suspected in patients with scaly eczematous plaques in skin folds. A high level of suspicion for micronutrient deficiencies in patients with IBD should be followed by a complete patient history, review of systems, and thorough clinical examination. A thorough laboratory evaluation can pinpoint nutritional deficiencies in patients with IBD, keeping in mind that specific biomarkers such as ferritin and serum zinc also act as acute phase reactants and should be interpreted in this context. Co-management with gastroenterologists should be a priority in patients with IBD, as gaining control of inflammatory disease is crucial for the prevention of recurrent vitamin and micronutrient deficiencies in addition to long-term health in this population.
In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.1 Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.2,3 Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.4 Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,5 offsetting this aversion to food can be difficult to overcome.2
Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B6 (pyridoxine), B9 (folic acid), and B12.6 Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.

Iron
A systematic review conducted from 2007 to 2012 in European patients with IBD (N=2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.7 Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.8
Pathophysiology—Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe3+ into enterocytes, where it is reduced to the transportable Fe2+.9,10 Distribution of Fe2+ ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.11 Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.12 This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.13
Cutaneous Manifestations—From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.14,15 Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency.
Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.16,17 Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).18 Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.19 An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (r=−0.768; P<.00001).20
Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.21 Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]=2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR=1.48; 95% CI, 0.32-6.82).22
Diagnosis and Monitoring—The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.23
Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.24 The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 µg/L in patients without evidence of active IBD and a ferritin level less than 100 µg/L for patients with active inflammation.25
A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 µg/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.26 In a sensitivity analysis stratifying patients by CRP level (<10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 µg/L (AUROC=0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC=0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 µg/L (AUROC=0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC=0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity >0.70) for iron deficiency in patients with Crohn disease.26
The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: r=0.66; P<.001).26 However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.27 Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration.
Treatment—Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR=2.32; P<.0001; IV: OR=3.05; P<.0001).28
Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.29 With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (P=.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.29
Zinc
A systematic review of zinc deficiency in patients with IBD identified 7 studies including 2413 patients and revealed those with Crohn disease had a higher prevalence of zinc deficiency compared with patients with ulcerative colitis (54% vs 41%).30
Pathophysiology—Zinc serves as a catalytic cofactor for enzymatic activity within proteins and immune cells.31 The homeostasis of zinc is tightly regulated within the brush border of the small intestine by zinc transporters ZIP4 and ZIP1 from the lumen of enterocytes into the bloodstream.32 Inflammation in the small intestine due to Crohn disease can result in zinc malabsorption.
Ranaldi et al33 exposed intestinal cells and zinc-depleted intestinal cells to tumor necrosis factor α media to simulate an inflammatory environment. They measured transepithelial electrical resistance as a surrogate for transmembrane permeability and found that zinc-depleted cells had a statistically significantly higher transepithelial electrical resistance percentage (60% reduction after 4 hours; P<1.10–6) when exposed to tumor necrosis factor α signaling compared with normal intestinal cells. They concluded that zinc deficiency can increase intestinal permeability in the presence of inflammation, creating a cycle of further nutrient malabsorption and inflammation exacerbating IBD symptoms.33
Cutaneous Manifestations—After absorption in the small intestine, approximately 5% of zinc resides in the skin, with the highest concentration in the stratum spinosum.34 A cell study found that keratinocytes in zinc-deficient environments had higher rates of apoptosis compared with cells in normal media. The authors proposed that this higher rate of apoptosis and the resulting inflammation could be a mechanism for developing the desquamative or eczematous scaly plaques that are common cutaneous manifestations of zinc deficiency.35
Other cutaneous findings may include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.36 The histopathology of these skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.37
Diagnosis and Monitoring—Assessing serum zinc levels is challenging, as they may decrease during states of inflammation.38 A mouse model study showed a 3.1-fold increase (P<.001) in ZIP14 expression in wild-type mice compared with an IL-6 -/- knock-down model after IL-6 exposure. The authors concluded that the upregulation of ZIP14 in the liver due to inflammatory cytokine upregulation decreases zinc availability in serum.39 Additionally, serum zinc can overestimate the level of deficiency in IBD because approximately 75% of serum zinc is bound to albumin, which decreases in the setting of inflammation.40-42
Alternatively, alkaline phosphatase (AP), a zinc-dependent metalloenzyme, may be a better evaluator of zinc status during periods of inflammation. A study in rats evaluated zinc through serum zinc levels and AP levels after a period of induced stress to mimic a short-term inflammatory state.43 The researchers found that total body stores of zinc were unaffected throughout the experiment; only serum zinc declined throughout the experiment duration while AP did not. Because approximately 75% of serum zinc is bound to serum albumin,42 the researchers concluded the induced inflammatory state depleted serum albumin and redistributed zinc to the liver, causing the observed serum zinc changes, while total body zinc levels and AP were largely unaffected in comparison.43 Comorbid conditions such as liver or bone disease can increase AP levels, which limits the utility of AP as a surrogate for zinc in patients with comorbidities.44 However, even in the context of active IBD, serum zinc still is currently considered the best biomarker to evaluate zinc status.45
Treatment—The recommended dose for zinc supplementation is 20 to 40 mg daily with higher doses (>50 mg/d) for patients with malabsorptive syndromes such as IBD.46 It can be administered orally or parenterally. Although rare, zinc replacement therapy may be associated with diarrhea, nausea, vomiting, mild headaches, and fatigue.46 Additional considerations should be taken when repleting other micronutrients with zinc, as calcium and folate can inhibit zinc reabsorption, while zinc itself can inhibit iron and copper reabsorption.47
Vitamin D and Calcium
Low vitamin D levels (<50 nmol/L) and hypocalcemia (<8.8 mg/dL) are common in patients with IBD.48,49
Pathophysiology—Vitamin D levels are maintained via 2 mechanisms. The first mechanism is through the skin, as keratinocytes produce 7-dehydrocholesterol after exposure to UV light, which is converted into previtamin D3 and then thermally isomerizes into vitamin D3. This vitamin D3 is then transported to the liver on vitamin D–binding protein.50 The second mechanism is through oral vitamin D3 that is absorbed through vitamin D receptors in intestinal epithelium and transported to the liver, where it is hydroxylated into 25-hydroxyvitamin D (25[OH]D), then to the kidneys for hydroxylation to 1,25(OH)2D for redistribution throughout the body.50 This activated form of vitamin D regulates calcium absorption in the intestine, and optimal vitamin D levels are necessary to absorb calcium efficiently.51 Inflammation from IBD within the small intestine can downregulate vitamin D receptors, causing malabsorption and decreased serum vitamin D.52
Vitamin D signaling also is vital to maintaining the tight junctions and adherens junctions of the intestinal epithelium. Weakening the permeability of the epithelium further exacerbates malabsorption and subsequent vitamin D deficiency.52 A meta-analysis of 27 studies including 8316 patients with IBD showed low vitamin D levels were associated with increased odds of disease activity (OR=1.53; 95% CI, 1.32-1.77), mucosal inflammation (OR=1.25; 95% CI, 1.06-1.47), and future clinical relapse (OR=1.23; 95% CI, 1.03-1.47) in patients with Crohn disease. The authors concluded that low levels of vitamin D could be used as a potential biomarker of inflammatory status in Crohn disease.53
Vitamin D and calcium are further implicated in maintaining skeletal health,47 while vitamin D specifically helps maintain intestinal homeostasis54 and immune system modulation in the skin.55
Cutaneous Manifestations—Vitamin D is thought to play crucial roles in skin differentiation and proliferation, cutaneous innate immunity, hair follicle cycling, photoprotection, and wound healing.56 Vitamin D deficiency has been observed in a large range of cutaneous diseases including skin cancer, psoriasis, vitiligo, bullous pemphigoid, atopic dermatitis, and various types of alopecia.56-59 It is unclear whether vitamin D deficiency facilitates these disease processes or is merely the consequence of a disrupted cutaneous surface with the inability to complete the first step in vitamin D processing. A 2014 meta-analysis of 290 prospective cohort studies and 172 randomized trials concluded that 25(OH)D deficiency was associated with ill health and did not find causal evidence for any specific disease, dermatologic or otherwise.60 Calcium deficiency may cause epidermal changes including dry skin, coarse hair, and brittle nails.61
Diagnosis and Monitoring—The ECCO guidelines recommend obtaining serum 25(OH)D levels every 3 months in patients with IBD.62 Levels less than 75 nmol/L are considered deficient, and a value less than 30 nmol/L increases the risk for osteomalacia and nutritional rickets, constituting severe vitamin D deficiency.63-65
An observational study of 325 patients with IBD showed a statistically significant negative correlation between serum vitamin D and fecal calprotectin (r=−0.19; P<.001), a stool-based marker for gut inflammation, supporting vitamin D as a potential biomarker in IBD.66
Evaluation of calcium can be done through serum levels in patients with IBD.67 Patients with IBD are at risk for hypoalbuminemia; therefore, consideration should be taken to ensure calcium levels are corrected, as approximately 50% of calcium is bound to albumin or other ions in the body,68 which can be done by adjusting the calcium concentration by 0.02 mmol/L for every 1 g/L of albumin above or below 40 g/L. In the most critically ill patients, a direct ionized calcium blood level should be used instead because the previously mentioned correction calculations are inaccurate when albumin is critically low.69
Treatment—The ECCO guidelines recommend calcium and vitamin D repletion of 500 to 1000 mg and 800 to 1000 U, respectively, in patients with IBD on systemic corticosteroids to prevent the negative effects of bone loss.62 Calcium repletion in patients with IBD who are not on systemic steroids are the same as for the general population.65
Vitamin D repletion also may help decrease IBD activity. In a prospective study, 10,000 IU/d of vitamin D in 10 patients with IBD—adjusted over 12 weeks to a target of 100 to 125 nmol/L of serum 25(OH)D—showed a significant reduction in clinical Crohn activity (P=.019) over the study period.70 In contrast, 2000 IU/d for 3 months in an RCT of 27 patients with Crohn disease found significantly lower CRP (P=.019) and significantly higher self-reported quality of life (P=.037) but nonsignificant decreases in Crohn activity (P=.082) in patients with 25(OH)D levels of 75 nmol/L or higher compared with those with 25(OH)D levels less than 75 nmol/L.71
These discrepancies illustrate the need for expanded clinical trials to elucidate the optimal vitamin D dosing for patients with IBD. Ultimately, assessing vitamin D and calcium status and considering repletion in patients with IBD, especially those with comorbid dermatologic diseases such as poor wound healing, psoriasis, or atopic dermatitis, is important.
Vitamin B6 (Pyridoxine)
Pathophysiology—Pyridoxine is an important coenzyme for many functions including amino acid transamination, fatty acid metabolism, and conversion of tryptophan to niacin. It is absorbed in the jejunum and ileum and subsequently transported to the liver for rephosphorylation and release into its active form.36 An observational study assessing the nutritional status of patients with IBD found that only 5.7% of 105 patients with food records had inadequate dietary intake of pyridoxine, but 29% of all patients with IBD had subnormal pyridoxine levels.72 Additionally, they found no significant difference in the prevalence of subnormal pyridoxine levels in patients with active IBD vs IBD in remission. The authors suggested that the subnormal pyridoxine levels in patients with IBD likely were multifactorial and resulted from malabsorption due to active disease, inflammation, and inadequate intake.72
Cutaneous Manifestations—Cutaneous findings associated with pyridoxine deficiency include periorificial and perineal dermatitis,73 angular stomatitis, and cheilitis with associated burning, redness, and tongue edema.36 Additionally, pyridoxine is involved in the conversion of tryptophan to niacin, and its deficiency may manifest with pellagralike findings.74
Because pyridoxine is critical to protein metabolism, its deficiency may disrupt key cellular structures that rely on protein concentrations to maintain structural integrity. One such structure in the skin that heavily relies on protein concentrations is the ground substance of the extracellular matrix—the amorphous gelatinous spaces that occupy the areas between the extracellular matrix, which consists of cross-linked glycosaminoglycans and proteins.75 Without protein, ground substance increases in viscosity and can disrupt the epidermal barrier, leading to increased transepidermal water loss and ultimately inflammation.76 Although this theory has yet to be validated fully, this is a potential mechanistic explanation for the inflammation in dermal papillae that leads to dermatitis observed in pyridoxine deficiency.
Diagnosis and Monitoring—Direct biomarkers of pyridoxine status are in serum, plasma, erythrocytes, and urine, with the most common measurement in plasma as pyridoxal 5′-phosphate (PLP).77 Plasma PLP concentrations lower than 20 nmol/L are suggestive of deficiency.78 Plasma PLP has shown inverse relationships with acute phase inflammatory markers CRP79 and AP,78 thereby raising concerns for its validity to assess pyridoxine status in patients with symptomatic IBD.80
Alternative evaluations of pyridoxine include tryptophan and methionine loading tests,36 which are measured via urinary excretion and require normal kidney function to be accurate. They should be considered in IBD if necessary, but routine testing, even in patients with symptomatic IBD, is not recommended in the ECCO guidelines. Additional considerations should be taken in patients with altered nutrient requirements such as those who have undergone bowel resection due to highly active disease or those who receive parenteral nutritional supplementation.81
Treatment—Recommendations for oral pyridoxine supplementation range from 25 to 600 mg daily,82 with symptoms typically improving on 100 mg daily.36 Pyridoxine supplementation may have additional benefits for patients with IBD and potentially modulate disease severity. An IL-10 knockout mouse supplemented with pyridoxine had an approximately 60% reduction (P<.05) in inflammation compared to mice deficient in pyridoxine.83 The authors suggest that PLP-dependent enzymes can inhibit further proinflammatory signaling and T-cell migration that can exacerbate IBD. Ultimately, more data is needed before determining the efficacy of pyridoxine supplementation for active IBD.
Vitamin B12 and Vitamin B9 (Folic Acid)
Pathophysiology—Vitamin B12 is reabsorbed in the terminal ileum, the distal portion of the small intestine. The American Gastroenterological Association recommends that patients with a history of extensive ileal disease or prior ileal surgery, which is the case for many patients with Crohn disease, be monitored for vitamin B12 deficiency.23 Monitoring and rapid supplementation of vitamin B12 can prevent pernicious anemia and irreversible neurologic damage that may result from deficiency.84
Folic acid is primarily absorbed in the duodenum and jejunum of the small intestine. A meta-analysis performed in 2017 assessed studies observing folic acid and vitamin B12 levels in 1086 patients with IBD compared with 1484 healthy controls and found an average difference in serum folate concentration of 0.46 nmol/L (P<.001).84 Interestingly, this study did not find a significant difference in serum vitamin B12 levels between patients with IBD and healthy controls, highlighting the mechanism of vitamin B12 deficiency in IBD because only patients with terminal ileal involvement are at risk for malabsorption and subsequent deficiency.
Cutaneous Manifestations—Both vitamin B12 and folic acid deficiency can manifest as cheilitis, glossitis, and/or generalized hyperpigmentation that is accentuated in the flexural areas, palms, soles, and oral cavity.85,86 Systemic symptoms of patients with vitamin B12 and folic acid deficiency include megaloblastic anemia, pallor, and fatigue. A potential mechanism for the hyperpigmentation observed from vitamin B12 deficiency came from an electron microscope study that showed an increased concentration of melanosomes in a patient with deficiency.87
Diagnosis and Monitoring—In patients with suspected vitamin B12 and/or folic acid deficiency, initial evaluation should include a CBC with peripheral smear and serum vitamin B12 and folate levels. In cases for which the diagnosis still is unclear after initial testing,
Treatment—According to the Centers for Disease Control and Prevention, supplementation of vitamin B12 can be done orally with 1000 µg daily in patients with deficiency. In patients with active IBD, oral reabsorption of vitamin B12 can be less effective, making subcutaneous or intramuscular administration (1000 µg/wk for 8 weeks, then monthly for life) better options.89
Patients with IBD managed with methotrexate should be screened carefully for folate deficiency. Methotrexate is a folate analog that sometimes is used for the treatment of IBD. Reversible competitive inhibition of dihydrofolate reductase can precipitate a systemic folic acid decrease.91 Typically, oral folic acid (1 to 5 mg/d) is sufficient to treat folate deficiency, with the ESPEN recommending 5 mg once weekly 24 to 72 hours after methotrexate treatment or 1 mg daily for 5 days per week in patients with IBD.1 Alternative formulations—IV, subcutaneous, or intramuscular—are available for patients who cannot tolerate oral intake.92
Final Thoughts
Dermatologists can be the first to observe the cutaneous manifestations of micronutrient deficiencies. Although the symptoms of each micronutrient deficiency discussed may overlap, attention to small clinical clues in patients with IBD can improve patient outcomes and quality of life. For example, koilonychia with glossitis and xerosis likely is due to iron deficiency, while zinc deficiency should be suspected in patients with scaly eczematous plaques in skin folds. A high level of suspicion for micronutrient deficiencies in patients with IBD should be followed by a complete patient history, review of systems, and thorough clinical examination. A thorough laboratory evaluation can pinpoint nutritional deficiencies in patients with IBD, keeping in mind that specific biomarkers such as ferritin and serum zinc also act as acute phase reactants and should be interpreted in this context. Co-management with gastroenterologists should be a priority in patients with IBD, as gaining control of inflammatory disease is crucial for the prevention of recurrent vitamin and micronutrient deficiencies in addition to long-term health in this population.
- Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin Nutr. 2023;42:352-379. doi:10.1016/j.clnu.2022.12.004
- Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammator y bowel disease. J Hum Nutr Diet. 2011;24:313-326. doi:10.1111/j.1365-277X.2011.01171.x
- Mentella MC, Scaldaferri F, Pizzoferrato M, et al. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020;12:944. doi:10.3390/nu12040944
- Bonsack O, Caron B, Baumann C, et al. Food avoidance and fasting in patients with inflammatory bowel disease: experience from the Nancy IBD nutrition clinic. United European Gastroenterol J. 2023;11:361-370. doi:10.1002/ueg2.1238521
- Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;13:1067. doi:10.3390/nu13041067
- Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. Clin Gastroenterol Hepatol. 2020;18:2645-2649.e4. doi:10.1016/j.cgh.2020.06.039
- Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. doi:10.1097/01.MIB.0000442728.74340.fd
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. doi:10.1038/nrgastro.2010.151
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls [Internet]. Updated April 17, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448204/
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. doi:10.1136/gut.2010.214312
- Przybyszewska J, Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol. 2014;9:208-213. doi:10.5114/pg.2014.45102
- Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175-178. doi:10.3324/haematol.2009.017046
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. doi:10.4291/wjgp.v6.i3.62
- Moiz B. Spoon nails: still seen in today’s world. Clin Case Rep. 2018;6:547-548. doi:10.1002/ccr3.1404
- St Pierre SA, Vercellotti GM, Donovan JC, et al. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070-1076. doi:10.1016/j.jaad.2009.05.054
- Chiang CP, Yu-Fong Chang J, Wang YP, et al. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in atrophic glossitis patients with or without microcytosis. J Formos Med Assoc. 2019;118:1401-1407. doi:10.1016/j.jfma.2019.06.004
- Chiang CP, Chang JY, Wang YP, et al. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J Formos Med Assoc. 2020;119:774-780. doi:10.1016/j.jfma.2019.04.015
- Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi:10.1111/jdv.13610
- Guo HF, Tsai CL, Terajima M, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding. Nat Commun. 2018;9:512. doi:10.1038/s41467-018-02859-z
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088-1092. doi:10.1126/science.1157121
- Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-477.e16. doi:10.1016/j.jaad.2018.07.013
- Hashash JG, Elkins J, Lewis JD, et al. AGA Clinical Practice Update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review [published online January 23, 2024]. Gastroenterology. doi:10.1053/j.gastro.2023.11.303
- Choudhuri S, Chowdhury IH, Saha A, et al. Acute monocyte pro- inflammatory response predicts higher positive to negative acute phase reactants ratio and severe hemostatic derangement in dengue fever. Cytokine. 2021;146:155644. doi:10.1016/j.cyto.2021.155644
- Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohn’s Colitis. 2015;9:211-222. doi:10.1093/ecco-jcc/jju009
- Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:1087-1095. doi:10.1111/apt.15739
- Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(suppl 6):1606S-1614S. doi:10.3945/ajcn.117.155887
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. doi:10.1371/journal.pone.0117383
- Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e8532. doi:10.1053/j.gastro.2011.06.005
- Zupo R, Sila A, Castellana F, et al. Prevalence of zinc deficiency in inflammatory bowel disease: a systematic review and meta-analysis. Nutrients. 2022;14:4052. doi:10.3390/nu14194052
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals. 2022;35:187-213. doi:10.1007/s10534-022-00373-w
- Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12:762. doi:10.3390/nu12030762
- Ranaldi G, Ferruzza S, Canali R, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967-976. doi:10.1016/j.jnutbio.2012.06.020
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016;611:113-119. doi:10.1016/j.abb.2016.06.003
- Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol. 2006;84:28-37. doi:10.1111/j.1440-1711.2005.01391.x
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685. doi:10.1016/j.clindermatol.2010.03.029
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi:10.3390/nu9060624
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843-6848. doi:10.1073/pnas.0502257102
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. Gut. 2006;55:426-431. doi:10.1136/gut.2005.069476
- Morisaku M, Ito K, Ogiso A, et al. Correlation between serum albumin and serum zinc in malignant lymphoma. Fujita Med J. 2022;8:59-64. doi:10.20407/fmj.2021-006
- Falchuk KH. Effect of acute disease and ACTH on serum zinc proteins. N Engl J Med. 1977:296:1129-1134.
- Naber TH, Baadenhuysen H, Jansen JB, et al. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin Chim Acta. 1996;249:109-127. doi:10.1016/0009-8981(96)06281-x
- Lowe D, Sanvictores T, Zubair M, et al. Alkaline phosphatase. StatPearls [Internet]. Updated October 29, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459201/
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 suppl 1:19-29. doi:10.1159/000348261
- Maxfield L, Shukla S, Crane JS. Zinc deficiency. StatPearls [Internet]. Updated June 28, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493231/
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808. doi:10.1016/j.gtc.2017.08.011
- Caviezel D, Maissen S, Niess JH, et al. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm Intest Dis. 2018;2:200-210. doi:10.1159/000489010
- Jasielska M, Grzybowska-Chlebowczyk U. Hypocalcemia and vitamin D deficiency in children with inflammatory bowel diseases and lactose intolerance. Nutrients. 2021;13:2583. doi:10.3390/nu13082583
- Vernia F, Valvano M, Longo S, et al. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients. 2022;14:269. doi:10.3390/nu14020269
- Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. doi:10.1007/s11926-008-0020-y
- Domazetovic V, Iantomasi T, Bonanomi AG, et al. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int J Colorectal Dis. 2020;35:1231-1242. doi:10.1007/s00384-020-03576-0
- Gubatan J, Chou ND, Nielsen OH, et al. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146-1158. doi:10.1111/apt.15506
- Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi:10.1016/j.jsbmb.2020.105663
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. doi:10.1126/science.1123933
- Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793-804. doi:10.1016/j.jare.2014.01.011
- Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397-409.
- Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43:529-535.
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404-412.
- Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. doi:10.1016/S2213-8587(13)70165-7
- Schafer AL, Shoback DM. Hypocalcemia: diagnosis and treatment. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. Updated January 3, 2016. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK279022/
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649-670. doi:10.1093/ecco-jcc/jjx008
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415. doi:10.1210/jc.2015-2175
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US); 2011.
- Yeaman F, Nguyen A, Abasszade J, et al. Assessing vitamin D as a biomarker in inflammatory bowel disease. JGH Open. 2023;7:953-958. doi:10.1002/jgh3.13010
- Vernia P, Loizos P, Di Giuseppantonio I, et al S. Dietary calcium intake in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:312-317. doi:10.1016/j.crohns.2013.09.008
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298-1302. doi:10.1136/bmj.39582.589433.BE
- Kenny CM, Murphy CE, Boyce DS, et al. Things we do for no reason™: calculating a “corrected calcium” level. J Hosp Med. 2021;16:499-501. doi:10.12788/jhm.3619
- Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr. 2018;37:1375-1382. doi:10.1016/j.clnu.2017.06.011
- Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294-302. doi:10.1177/2050640615572176
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. doi:10.1177/0148607107031004311
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274. doi:10.1016/s0190-9622(86)70301-0
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739. doi:10.3949/ccjm.83a.15061
- Elgharably N, Al Abadie M, Al Abadie M, et al. Vitamin B group levels and supplementations in dermatology. Dermatol Reports. 2022;15:9511. doi:10.4081/dr.2022.9511
- Hołubiec P, Leon´czyk M, Staszewski F, et al. Pathophysiology and clinical management of pellagra—a review. Folia Med Cracov. 2021;61:125-137. doi:10.24425/fmc.2021.138956
- Ink SL, Henderson LM. Vitamin B6 metabolism. Annu Rev Nutr. 1984;4:455-470. doi:10.1146/annurev.nu.04.070184.002323
- Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 deficiency. StatPearls [Internet]. Updated August 8, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470579/.
- Vasilaki AT, McMillan DC, Kinsella J, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr. 2008;88:140-146. doi:10.1093/ajcn/88.1.140
- Chiang EP, Bagley PJ, Selhub J, et al. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283-287. doi:10.1016/s0002-9343(02)01528-0
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. doi:10.1093/ecco-jcc/jjy113
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138-2143. doi:10.1016/j.jnutbio.2013.08.005
- Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. doi:10.3390/nu9040382
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33. doi:10.1007/s40257-014-0107-3
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503. doi:10.1002/ncp.10321
- Mori K, Ando I, Kukita A. Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J Dermatol. 2001;28:282-285. doi:10.1111/j.1346-8138.2001.tb00134.x
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011;94:666S-672S. doi:10.3945/ajcn.110.009613
- NIH Office of Dietary Supplements. Vitamin B12: fact sheet for health professionals. Updated February 27, 2024. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
- NIH Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 20, 2023. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.
- Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123-127. doi:10.1016/j.dld.2011.09.015
- Khan KM, Jialal I. Folic acid deficiency. StatPearls [Internet]. Updated June 26, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535377/
- Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin Nutr. 2023;42:352-379. doi:10.1016/j.clnu.2022.12.004
- Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammator y bowel disease. J Hum Nutr Diet. 2011;24:313-326. doi:10.1111/j.1365-277X.2011.01171.x
- Mentella MC, Scaldaferri F, Pizzoferrato M, et al. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020;12:944. doi:10.3390/nu12040944
- Bonsack O, Caron B, Baumann C, et al. Food avoidance and fasting in patients with inflammatory bowel disease: experience from the Nancy IBD nutrition clinic. United European Gastroenterol J. 2023;11:361-370. doi:10.1002/ueg2.1238521
- Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;13:1067. doi:10.3390/nu13041067
- Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. Clin Gastroenterol Hepatol. 2020;18:2645-2649.e4. doi:10.1016/j.cgh.2020.06.039
- Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. doi:10.1097/01.MIB.0000442728.74340.fd
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. doi:10.1038/nrgastro.2010.151
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls [Internet]. Updated April 17, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448204/
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. doi:10.1136/gut.2010.214312
- Przybyszewska J, Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Prz Gastroenterol. 2014;9:208-213. doi:10.5114/pg.2014.45102
- Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175-178. doi:10.3324/haematol.2009.017046
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. doi:10.4291/wjgp.v6.i3.62
- Moiz B. Spoon nails: still seen in today’s world. Clin Case Rep. 2018;6:547-548. doi:10.1002/ccr3.1404
- St Pierre SA, Vercellotti GM, Donovan JC, et al. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070-1076. doi:10.1016/j.jaad.2009.05.054
- Chiang CP, Yu-Fong Chang J, Wang YP, et al. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in atrophic glossitis patients with or without microcytosis. J Formos Med Assoc. 2019;118:1401-1407. doi:10.1016/j.jfma.2019.06.004
- Chiang CP, Chang JY, Wang YP, et al. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J Formos Med Assoc. 2020;119:774-780. doi:10.1016/j.jfma.2019.04.015
- Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi:10.1111/jdv.13610
- Guo HF, Tsai CL, Terajima M, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding. Nat Commun. 2018;9:512. doi:10.1038/s41467-018-02859-z
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088-1092. doi:10.1126/science.1157121
- Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-477.e16. doi:10.1016/j.jaad.2018.07.013
- Hashash JG, Elkins J, Lewis JD, et al. AGA Clinical Practice Update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review [published online January 23, 2024]. Gastroenterology. doi:10.1053/j.gastro.2023.11.303
- Choudhuri S, Chowdhury IH, Saha A, et al. Acute monocyte pro- inflammatory response predicts higher positive to negative acute phase reactants ratio and severe hemostatic derangement in dengue fever. Cytokine. 2021;146:155644. doi:10.1016/j.cyto.2021.155644
- Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohn’s Colitis. 2015;9:211-222. doi:10.1093/ecco-jcc/jju009
- Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:1087-1095. doi:10.1111/apt.15739
- Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(suppl 6):1606S-1614S. doi:10.3945/ajcn.117.155887
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. doi:10.1371/journal.pone.0117383
- Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e8532. doi:10.1053/j.gastro.2011.06.005
- Zupo R, Sila A, Castellana F, et al. Prevalence of zinc deficiency in inflammatory bowel disease: a systematic review and meta-analysis. Nutrients. 2022;14:4052. doi:10.3390/nu14194052
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals. 2022;35:187-213. doi:10.1007/s10534-022-00373-w
- Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12:762. doi:10.3390/nu12030762
- Ranaldi G, Ferruzza S, Canali R, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967-976. doi:10.1016/j.jnutbio.2012.06.020
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016;611:113-119. doi:10.1016/j.abb.2016.06.003
- Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol. 2006;84:28-37. doi:10.1111/j.1440-1711.2005.01391.x
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685. doi:10.1016/j.clindermatol.2010.03.029
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi:10.3390/nu9060624
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843-6848. doi:10.1073/pnas.0502257102
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. Gut. 2006;55:426-431. doi:10.1136/gut.2005.069476
- Morisaku M, Ito K, Ogiso A, et al. Correlation between serum albumin and serum zinc in malignant lymphoma. Fujita Med J. 2022;8:59-64. doi:10.20407/fmj.2021-006
- Falchuk KH. Effect of acute disease and ACTH on serum zinc proteins. N Engl J Med. 1977:296:1129-1134.
- Naber TH, Baadenhuysen H, Jansen JB, et al. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin Chim Acta. 1996;249:109-127. doi:10.1016/0009-8981(96)06281-x
- Lowe D, Sanvictores T, Zubair M, et al. Alkaline phosphatase. StatPearls [Internet]. Updated October 29, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459201/
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 suppl 1:19-29. doi:10.1159/000348261
- Maxfield L, Shukla S, Crane JS. Zinc deficiency. StatPearls [Internet]. Updated June 28, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493231/
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808. doi:10.1016/j.gtc.2017.08.011
- Caviezel D, Maissen S, Niess JH, et al. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm Intest Dis. 2018;2:200-210. doi:10.1159/000489010
- Jasielska M, Grzybowska-Chlebowczyk U. Hypocalcemia and vitamin D deficiency in children with inflammatory bowel diseases and lactose intolerance. Nutrients. 2021;13:2583. doi:10.3390/nu13082583
- Vernia F, Valvano M, Longo S, et al. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients. 2022;14:269. doi:10.3390/nu14020269
- Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. doi:10.1007/s11926-008-0020-y
- Domazetovic V, Iantomasi T, Bonanomi AG, et al. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int J Colorectal Dis. 2020;35:1231-1242. doi:10.1007/s00384-020-03576-0
- Gubatan J, Chou ND, Nielsen OH, et al. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146-1158. doi:10.1111/apt.15506
- Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi:10.1016/j.jsbmb.2020.105663
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. doi:10.1126/science.1123933
- Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793-804. doi:10.1016/j.jare.2014.01.011
- Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397-409.
- Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43:529-535.
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404-412.
- Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. doi:10.1016/S2213-8587(13)70165-7
- Schafer AL, Shoback DM. Hypocalcemia: diagnosis and treatment. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. Updated January 3, 2016. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK279022/
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649-670. doi:10.1093/ecco-jcc/jjx008
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415. doi:10.1210/jc.2015-2175
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US); 2011.
- Yeaman F, Nguyen A, Abasszade J, et al. Assessing vitamin D as a biomarker in inflammatory bowel disease. JGH Open. 2023;7:953-958. doi:10.1002/jgh3.13010
- Vernia P, Loizos P, Di Giuseppantonio I, et al S. Dietary calcium intake in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:312-317. doi:10.1016/j.crohns.2013.09.008
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298-1302. doi:10.1136/bmj.39582.589433.BE
- Kenny CM, Murphy CE, Boyce DS, et al. Things we do for no reason™: calculating a “corrected calcium” level. J Hosp Med. 2021;16:499-501. doi:10.12788/jhm.3619
- Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr. 2018;37:1375-1382. doi:10.1016/j.clnu.2017.06.011
- Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294-302. doi:10.1177/2050640615572176
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. doi:10.1177/0148607107031004311
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274. doi:10.1016/s0190-9622(86)70301-0
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739. doi:10.3949/ccjm.83a.15061
- Elgharably N, Al Abadie M, Al Abadie M, et al. Vitamin B group levels and supplementations in dermatology. Dermatol Reports. 2022;15:9511. doi:10.4081/dr.2022.9511
- Hołubiec P, Leon´czyk M, Staszewski F, et al. Pathophysiology and clinical management of pellagra—a review. Folia Med Cracov. 2021;61:125-137. doi:10.24425/fmc.2021.138956
- Ink SL, Henderson LM. Vitamin B6 metabolism. Annu Rev Nutr. 1984;4:455-470. doi:10.1146/annurev.nu.04.070184.002323
- Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 deficiency. StatPearls [Internet]. Updated August 8, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470579/.
- Vasilaki AT, McMillan DC, Kinsella J, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr. 2008;88:140-146. doi:10.1093/ajcn/88.1.140
- Chiang EP, Bagley PJ, Selhub J, et al. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283-287. doi:10.1016/s0002-9343(02)01528-0
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. doi:10.1093/ecco-jcc/jjy113
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138-2143. doi:10.1016/j.jnutbio.2013.08.005
- Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. doi:10.3390/nu9040382
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33. doi:10.1007/s40257-014-0107-3
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503. doi:10.1002/ncp.10321
- Mori K, Ando I, Kukita A. Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J Dermatol. 2001;28:282-285. doi:10.1111/j.1346-8138.2001.tb00134.x
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011;94:666S-672S. doi:10.3945/ajcn.110.009613
- NIH Office of Dietary Supplements. Vitamin B12: fact sheet for health professionals. Updated February 27, 2024. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
- NIH Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 20, 2023. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.
- Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123-127. doi:10.1016/j.dld.2011.09.015
- Khan KM, Jialal I. Folic acid deficiency. StatPearls [Internet]. Updated June 26, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535377/
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>Le 0424</fileName> <TBEID>0C02F4BB.SIG</TBEID> <TBUniqueIdentifier>NJ_0C02F4BB</TBUniqueIdentifier> <newsOrJournal>Journal</newsOrJournal> <publisherName>Frontline Medical Communications Inc.</publisherName> <storyname>Micronutrient Deficiencies in Pa</storyname> <articleType>1</articleType> <TBLocation>Copyfitting-CT</TBLocation> <QCDate/> <firstPublished>20240408T093751</firstPublished> <LastPublished>20240408T093751</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240408T093751</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Todd A. Le, MS; Sumona Saha, MD, MS; Bridget E. Shields, MD</byline> <bylineText>Todd A. Le, MS; Sumona Saha, MD, MS; Bridget E. Shields, MD</bylineText> <bylineFull>Todd A. Le, MS; Sumona Saha, MD, MS; Bridget E. Shields, MD</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>(choose one)</newsDocType> <journalDocType>(choose one)</journalDocType> <linkLabel/> <pageRange>159-166</pageRange> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:"> <name/> <rightsInfo> <copyrightHolder> <name/> </copyrightHolder> <copyrightNotice/> </rightsInfo> </provider> <abstract/> <metaDescription>In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitorin</metaDescription> <articlePDF>300905</articlePDF> <teaserImage/> <title>Micronutrient Deficiencies in Patients With Inflammatory Bowel Disease</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth>April</pubPubdateMonth> <pubPubdateDay/> <pubVolume>113</pubVolume> <pubNumber>4</pubNumber> <wireChannels/> <primaryCMSID/> <CMSIDs> <CMSID>2165</CMSID> </CMSIDs> <keywords> <keyword>inflammatory bowel disease</keyword> </keywords> <seeAlsos/> <publications_g> <publicationData> <publicationCode>CT</publicationCode> <pubIssueName>April 2024</pubIssueName> <pubArticleType>Audio | 2165</pubArticleType> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Cutis</journalTitle> <journalFullTitle>Cutis</journalFullTitle> <copyrightStatement>Copyright 2015 Frontline Medical Communications Inc., Parsippany, NJ, USA. All rights reserved.</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">12</term> </publications> <sections> <term canonical="true">72605</term> </sections> <topics> <term canonical="true">199</term> </topics> <links> <link> <itemClass qcode="ninat:composite"/> <altRep contenttype="application/pdf">images/180026f7.pdf</altRep> <description role="drol:caption"/> <description role="drol:credit"/> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Micronutrient Deficiencies in Patients With Inflammatory Bowel Disease</title> <deck/> </itemMeta> <itemContent> <p class="abstract">Inflammatory bowel disease (IBD) can cause micronutrient deficiencies that have cutaneous manifestations. Dermatologists may be the first to identify an undiagnosed micronutrient deficiency in the affected population. The approach to monitoring and repleting a micronutrient deficiency may be impacted by factors such as IBD activity and potential interactions between supplements and medications used to treat IBD. In this article, we review the most common micronutrient deficiencies observed in patients with IBD and their associated cutaneous manifestations. We also provide guidance for monitoring and supplementing each micronutrient discussed.</p> <p>In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.<sup>1</sup> Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.<sup>2,3</sup> Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.<sup>4</sup> Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,<sup>5</sup> offsetting this aversion to food can be difficult to overcome.<sup>2</sup> </p> <p>Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B<sub>6</sub> (pyridoxine), B<sub>9</sub> (folic acid), and B<sub>12</sub>.<sup>6</sup> Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.</p> <h3>Iron</h3> <p>A systematic review conducted from 2007 to 2012 in European patients with IBD (N<span class="body">=</span>2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.<sup>7</sup> Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.<sup>8</sup> </p> <p><i>Pathophysiology—</i>Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe<sup>3+</sup> into enterocytes, where it is reduced to the transportable Fe<sup>2+</sup>.<sup>9,10</sup> Distribution of Fe<sup>2+</sup> ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.<sup>11</sup> Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.<sup>12</sup> This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.<sup>13</sup> <br/><br/><i>Cutaneous Manifestations—</i>From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.<sup>14,15</sup> Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency. <br/><br/>Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.<sup>16,17</sup> Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).<sup>18</sup> Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.<sup>19</sup> An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (<i>r</i><span class="body">=−</span>0.768; <i>P</i><span class="body"><</span>.00001).<sup>20</sup> <br/><br/>Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.<sup>21</sup> Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]<span class="body">=</span>2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR<span class="body">=</span>1.48; 95% CI, 0.32-6.82).<sup>22</sup> <br/><br/><i>Diagnosis and Monitoring—</i>The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.<sup>23</sup> <br/><br/>Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.<sup>24</sup> The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 <span class="body">µ</span>g/L in patients without evidence of active IBD and a ferritin level less than 100 <span class="body">µ</span>g/L for patients with active inflammation.<sup>25</sup> <br/><br/>A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 <span class="body">µ</span>g/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.<sup>26</sup> In a sensitivity analysis stratifying patients by CRP level (<span class="body"><</span>10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 <span class="body">µ</span>g/L (AUROC<span class="body">=</span>0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC<span class="body">=</span>0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 <span class="body">µ</span>g/L (AUROC<span class="body">=</span>0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC<span class="body">=</span>0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity <span class="body">></span>0.70) for iron deficiency in patients with Crohn disease.<sup>26</sup> <br/><br/>The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: <i>r</i><span class="body">=</span>0.66; <i>P</i><span class="body"><</span>.001).<sup>26</sup> However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.<sup>27</sup> Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration. <br/><br/><i>Treatment—</i>Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR<span class="body">=</span>2.32; <i>P</i><span class="body"><</span>.0001; IV: OR<span class="body">=</span>3.05; <i>P</i><span class="body"><</span>.0001).<sup>28</sup> <br/><br/>Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.<sup>29</sup> With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (<i>P</i><span class="body">=</span>.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.<sup>29</sup></p> <h3>Zinc</h3> <p>A systematic review of zinc deficiency in patients with IBD identified 7 studies including 2413 patients and revealed those with Crohn disease had a higher prevalence of zinc deficiency compared with patients with ulcerative colitis (54% vs 41%).<sup>30</sup> </p> <p><i>Pathophysiology—</i>Zinc serves as a catalytic cofactor for enzymatic activity within proteins and immune cells.<sup>31</sup> The homeostasis of zinc is tightly regulated within the brush border of the small intestine by zinc transporters ZIP4 and ZIP1 from the lumen of enterocytes into the bloodstream.<sup>32</sup> Inflammation in the small intestine due to Crohn disease can result in zinc malabsorption. <br/><br/>Ranaldi et al<sup>33</sup> exposed intestinal cells and zinc-depleted intestinal cells to tumor necrosis factor α media to simulate an inflammatory environment. They measured transepithelial electrical resistance as a surrogate for transmembrane permeability and found that zinc-depleted cells had a statistically significantly higher transepithelial electrical resistance percentage (60% reduction after 4 hours; <i>P</i><span class="body"><</span>1.10<sup>–6</sup>) when exposed to tumor necrosis factor α signaling compared with normal intestinal cells. They concluded that zinc deficiency can increase intestinal permeability in the presence of inflammation, creating a cycle of further nutrient malabsorption and inflammation exacerbating IBD symptoms.<sup>33</sup> <br/><br/><i>Cutaneous Manifestations—</i>After absorption in the small intestine, approximately 5% of zinc resides in the skin, with the highest concentration in the stratum spinosum.<sup>34</sup> A cell study found that keratinocytes in zinc-deficient environments had higher rates of apoptosis compared with cells in normal media. The authors proposed that this higher rate of apoptosis and the resulting inflammation could be a mechanism for developing the desquamative or eczematous scaly plaques that are common cutaneous manifestations of zinc deficiency.<sup>35<br/><br/></sup>Other cutaneous findings may include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.<sup>36</sup> The histopathology of these skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.<sup>37</sup> <br/><br/><i>Diagnosis and Monitoring—</i>Assessing serum zinc levels is challenging, as they may decrease during states of inflammation.<sup>38</sup> A mouse model study showed a 3.1-fold increase (<i>P</i><span class="body"><</span>.001) in ZIP14 expression in wild-type mice compared with an IL-6 -/- knock-down model after IL-6 exposure. The authors concluded that the upregulation of ZIP14 in the liver due to inflammatory cytokine upregulation decreases zinc availability in serum.<sup>39</sup> Additionally, serum zinc can overestimate the level of deficiency in IBD because approximately 75% of serum zinc is bound to albumin, which decreases in the setting of inflammation.<sup>40-42</sup> <br/><br/>Alternatively, alkaline phosphatase (AP), a zinc-dependent metalloenzyme, may be a better evaluator of zinc status during periods of inflammation. A study in rats evaluated zinc through serum zinc levels and AP levels after a period of induced stress to mimic a short-term inflammatory state.<sup>43</sup> The researchers found that total body stores of zinc were unaffected throughout the experiment; only serum zinc declined throughout the experiment duration while AP did not. Because approximately 75% of serum zinc is bound to serum albumin,<sup>42</sup> the researchers concluded the induced inflammatory state depleted serum albumin and redistributed zinc to the liver, causing the observed serum zinc changes, while total body zinc levels and AP were largely unaffected in comparison.<sup>43</sup> Comorbid conditions such as liver or bone disease can increase AP levels, which limits the utility of AP as a surrogate for zinc in patients with comorbidities.<sup>44</sup> However, even in the context of active IBD, serum zinc still is currently considered the best biomarker to evaluate zinc status.<sup>45</sup> <br/><br/><i>Treatment—</i>The recommended dose for zinc supplementation is 20 to 40 mg daily with higher doses (<span class="body">></span>50 mg/d) for patients with malabsorptive syndromes such as IBD.<sup>46</sup> It can be administered orally or parenterally. Although rare, zinc replacement therapy may be associated with diarrhea, nausea, vomiting, mild headaches, and fatigue.<sup>46</sup> Additional considerations should be taken when repleting other micronutrients with zinc, as calcium and folate can inhibit zinc reabsorption, while zinc itself can inhibit iron and copper reabsorption.<sup>47</sup> </p> <h3>Vitamin D and Calcium</h3> <p>Low vitamin D levels (<span class="body"><</span>50 nmol/L) and hypocalcemia (<span class="body"><</span>8.8 mg/dL) are common in patients with IBD.<sup>48,49</sup> </p> <p><i>Pathophysiology—</i>Vitamin D levels are maintained via 2 mechanisms. The first mechanism is through the skin, as keratinocytes produce 7-dehydrocholesterol after exposure to UV light, which is converted into previtamin D<sub>3</sub> and then thermally isomerizes into vitamin D<sub>3</sub>. This vitamin D<sub>3</sub> is then transported to the liver on vitamin D–binding protein.<sup>50</sup> The second mechanism is through oral vitamin D<sub>3</sub> that is absorbed through vitamin D receptors in intestinal epithelium and transported to the liver, where it is hydroxylated into 25-hydroxyvitamin D (25[OH]D), then to the kidneys for hydroxylation to 1,25(OH)<sub>2</sub>D for redistribution throughout the body.<sup>50</sup> This activated form of vitamin D regulates calcium absorption in the intestine, and optimal vitamin D levels are necessary to absorb calcium efficiently.<sup>51</sup> Inflammation from IBD within the small intestine can downregulate vitamin D receptors, causing malabsorption and decreased serum vitamin D.<sup>52</sup> <br/><br/>Vitamin D signaling also is vital to maintaining the tight junctions and adherens junctions of the intestinal epithelium. Weakening the permeability of the epithelium further exacerbates malabsorption and subsequent vitamin D deficiency.<sup>52</sup> A meta-analysis of 27 studies including 8316 patients with IBD showed low vitamin D levels were associated with increased odds of disease activity (OR<span class="body">=</span>1.53; 95% CI, 1.32-1.77), mucosal inflammation (OR<span class="body">=</span>1.25; 95% CI, 1.06-1.47), and future clinical relapse (OR<span class="body">=</span>1.23; 95% CI, 1.03-1.47) in patients with Crohn disease. The authors concluded that low levels of vitamin D could be used as a potential biomarker of inflammatory status in Crohn disease.<sup>53</sup> <br/><br/>Vitamin D and calcium are further implicated in maintaining skeletal health,<sup>47</sup> while vitamin D specifically helps maintain intestinal homeostasis<sup>54</sup> and immune system modulation in the skin.<sup>55</sup> <br/><br/><i>Cutaneous Manifestations—</i>Vitamin D is thought to play crucial roles in skin differentiation and proliferation, cutaneous innate immunity, hair follicle cycling, photoprotection, and wound healing.<sup>56</sup> Vitamin D deficiency has been observed in a large range of cutaneous diseases including skin cancer, psoriasis, vitiligo, bullous pemphigoid, atopic dermatitis, and various types of alopecia.<sup>56-59</sup> It is unclear whether vitamin D deficiency facilitates these disease processes or is merely the consequence of a disrupted cutaneous surface with the inability to complete the first step in vitamin D processing. A 2014 meta-analysis of 290 prospective cohort studies and 172 randomized trials concluded that 25(OH)D deficiency was associated with ill health and did not find causal evidence for any specific disease, dermatologic or otherwise.<sup>60</sup> Calcium deficiency may cause epidermal changes including dry skin, coarse hair, and brittle nails.<sup>61<br/><br/></sup><i>Diagnosis and Monitoring—</i>The ECCO guidelines recommend obtaining serum 25(OH)D levels every 3 months in patients with IBD.<sup>62</sup> Levels less than 75 nmol/L are considered deficient, and a value less than 30 nmol/L increases the risk for osteomalacia and nutritional rickets, constituting severe vitamin D deficiency.<sup>63-65</sup> <br/><br/>An observational study of 325 patients with IBD showed a statistically significant negative correlation between serum vitamin D and fecal calprotectin (<i>r</i><span class="body">=−</span>0.19; <i>P</i><span class="body"><</span>.001), a stool-based marker for gut inflammation, supporting vitamin D as a potential biomarker in IBD.<sup>66</sup> <br/><br/>Evaluation of calcium can be done through serum levels in patients with IBD.<sup>67</sup> Patients with IBD are at risk for hypoalbuminemia; therefore, consideration should be taken to ensure calcium levels are corrected, as approximately 50% of calcium is bound to albumin or other ions in the body,<sup>68</sup> which can be done by adjusting the calcium concentration by 0.02 mmol/L for every 1 g/L of albumin above or below 40 g/L. In the most critically ill patients, a direct ionized calcium blood level should be used instead because the previously mentioned correction calculations are inaccurate when albumin is critically low.<sup>69</sup> <br/><br/><i>Treatment—</i>The ECCO guidelines recommend calcium and vitamin D repletion of 500 to 1000 mg and 800 to 1000 U, respectively, in patients with IBD on systemic corticosteroids to prevent the negative effects of bone loss.<sup>62</sup> Calcium repletion in patients with IBD who are not on systemic steroids are the same as for the general population.<sup>65</sup> <br/><br/>Vitamin D repletion also may help decrease IBD activity. In a prospective study, 10,000 IU/d of vitamin D in 10 patients with IBD—adjusted over 12 weeks to a target of 100 to 125 nmol/L of serum 25(OH)D—showed a significant reduction in clinical Crohn activity (<i>P</i><span class="body">=</span>.019) over the study period.<sup>70</sup> In contrast, 2000 IU/d for 3 months in an RCT of 27 patients with Crohn disease found significantly lower CRP (<i>P</i><span class="body">=</span>.019) and significantly higher self-reported quality of life (<i>P</i><span class="body">=</span>.037) but nonsignificant decreases in Crohn activity (<i>P</i><span class="body">=</span>.082) in patients with 25(OH)D levels of 75 nmol/L or higher compared with those with 25(OH)D levels less than 75 nmol/L.<sup>71</sup> <br/><br/>These discrepancies illustrate the need for expanded clinical trials to elucidate the optimal vitamin D dosing for patients with IBD. Ultimately, assessing vitamin D and calcium status and considering repletion in patients with IBD, especially those with comorbid dermatologic diseases such as poor wound healing, psoriasis, or atopic dermatitis, is important. </p> <h3>Vitamin B<sub>6</sub> (Pyridoxine)</h3> <p><i>Pathophysiology—</i>Pyridoxine is an important coenzyme for many functions including amino acid transamination, fatty acid metabolism, and conversion of tryptophan to niacin. It is absorbed in the jejunum and ileum and subsequently transported to the liver for rephosphorylation and release into its active form.<sup>36</sup> An observational study assessing the nutritional status of patients with IBD found that only 5.7% of 105 patients with food records had inadequate dietary intake of pyridoxine, but 29% of all patients with IBD had subnormal pyridoxine levels.<sup>72</sup> Additionally, they found no significant difference in the prevalence of subnormal pyridoxine levels in patients with active IBD vs IBD in remission. The authors suggested that the subnormal pyridoxine levels in patients with IBD likely were multifactorial and resulted from malabsorption due to active disease, inflammation, and inadequate intake.<sup>72</sup></p> <p><i>Cutaneous Manifestations—</i>Cutaneous findings associated with pyridoxine deficiency include periorificial and perineal dermatitis,<sup>73</sup> angular stomatitis, and cheilitis with associated burning, redness, and tongue edema.<sup>36</sup> Additionally, pyridoxine is involved in the conversion of tryptophan to niacin, and its deficiency may manifest with pellagralike findings.<sup>74</sup> <br/><br/>Because pyridoxine is critical to protein metabolism, its deficiency may disrupt key cellular structures that rely on protein concentrations to maintain structural integrity. One such structure in the skin that heavily relies on protein concentrations is the ground substance of the extracellular matrix—the amorphous gelatinous spaces that occupy the areas between the extracellular matrix, which consists of cross-linked glycosaminoglycans and proteins.<sup>75</sup> Without protein, ground substance increases in viscosity and can disrupt the epidermal barrier, leading to increased transepidermal water loss and ultimately inflammation.<sup>76</sup> Although this theory has yet to be validated fully, this is a potential mechanistic explanation for the inflammation in dermal papillae that leads to dermatitis observed in pyridoxine deficiency. <br/><br/><i>Diagnosis and Monitoring—</i>Direct biomarkers of pyridoxine status are in serum, plasma, erythrocytes, and urine, with the most common measurement in plasma as pyridoxal 5′-phosphate (PLP).<sup>77</sup> Plasma PLP concentrations lower than 20 nmol/L are suggestive of deficiency.<sup>78</sup> Plasma PLP has shown inverse relationships with acute phase inflammatory markers CRP<sup>79</sup> and AP,<sup>78</sup> thereby raising concerns for its validity to assess pyridoxine status in patients with symptomatic IBD.<sup>80</sup> <br/><br/>Alternative evaluations of pyridoxine include tryptophan and methionine loading tests,<sup>36</sup> which are measured via urinary excretion and require normal kidney function to be accurate. They should be considered in IBD if necessary, but routine testing, even in patients with symptomatic IBD, is not recommended in the ECCO guidelines. Additional considerations should be taken in patients with altered nutrient requirements such as those who have undergone bowel resection due to highly active disease or those who receive parenteral nutritional supplementation.<sup>81</sup> <br/><br/><i>Treatment—</i>Recommendations for oral pyridoxine supplementation range from 25 to 600 mg daily,<sup>82</sup> with symptoms typically improving on 100 mg daily.<sup>36</sup> Pyridoxine supplementation may have additional benefits for patients with IBD and potentially modulate disease severity. An IL-10 knockout mouse supplemented with pyridoxine had an approximately 60% reduction (<i>P</i><span class="body"><</span>.05) in inflammation compared to mice deficient in pyridoxine.<sup>83</sup> The authors suggest that PLP-dependent enzymes can inhibit further proinflammatory signaling and T-cell migration that can exacerbate IBD. Ultimately, more data is needed before determining the efficacy of pyridoxine supplementation for active IBD.</p> <h3>Vitamin B<sub>12</sub> and Vitamin B<sub>9</sub> (Folic Acid)</h3> <p><i>Pathophysiology—</i>Vitamin B<sub>12</sub> is reabsorbed in the terminal ileum, the distal portion of the small intestine. The American Gastroenterological Association recommends that patients with a history of extensive ileal disease or prior ileal surgery, which is the case for many patients with Crohn disease, be monitored for vitamin B<sub>12</sub> deficiency.<sup>23</sup> Monitoring and rapid supplementation of vitamin B<sub>12</sub> can prevent pernicious anemia and irreversible neurologic damage that may result from deficiency.<sup>84</sup> </p> <p>Folic acid is primarily absorbed in the duodenum and jejunum of the small intestine. A meta-analysis performed in 2017 assessed studies observing folic acid and vitamin B<sub>12</sub> levels in 1086 patients with IBD compared with 1484 healthy controls and found an average difference in serum folate concentration of 0.46 nmol/L (<i>P</i><span class="body"><</span>.001).<sup>84</sup> Interestingly, this study did not find a significant difference in serum vitamin B<sub>12</sub> levels between patients with IBD and healthy controls, highlighting the mechanism of vitamin B<sub>12</sub> deficiency in IBD because only patients with terminal ileal involvement are at risk for malabsorption and subsequent deficiency.<br/><br/><i>Cutaneous Manifestations—</i>Both vitamin B<sub>12</sub> and folic acid deficiency can manifest as cheilitis, glossitis, and/or generalized hyperpigmentation that is accentuated in the flexural areas, palms, soles, and oral cavity.<sup>85,86</sup> Systemic symptoms of patients with vitamin B<sub>12</sub> and folic acid deficiency include megaloblastic anemia, pallor, and fatigue. A potential mechanism for the hyperpigmentation observed from vitamin B<sub>12</sub> deficiency came from an electron microscope study that showed an increased concentration of melanosomes in a patient with deficiency.<sup>87<br/><br/></sup><i>Diagnosis and Monitoring—</i>In patients with suspected vitamin B<sub>12</sub> and/or folic acid deficiency, initial evaluation should include a CBC with peripheral smear and serum vitamin B<sub>12</sub> and folate levels. In cases for which the diagnosis still is unclear after initial testing, <hl name="17870"/>methylmalonic acid and homocysteine levels can help differentiate between the 2 deficiencies. Methylmalonic acid classically is elevated (<span class="body">></span>260 nmol/L) in vitamin B<sub>12</sub> deficiency but not in folate deficiency.<sup>88</sup> Cut-off values for vitamin B<sub>12</sub> deficiency are less than 200 to 250 pg/mL forserum vitamin B<sub>12</sub> and/or an elevated level of methylmalonic acid (<span class="body">></span>0.271 <span class="body">µ</span>mol/L).<sup>89</sup> A serum folic acid value greater than 3 ng/mL and/or erythrocyte folate concentrations greater than 140 ng/mL are considered adequate, whereas an indicator of folic acid deficiency is a homocysteine level less than 10 <span class="body">µ</span>mol/L.<sup>90</sup> A CBC can screen for macrocytic megaloblastic anemias (mean corpuscular volume <span class="body">></span>100 fl), which are classic diagnostic signs of an underlying vitamin B<sub>12</sub> or folate deficiency. <br/><br/><i>Treatment—</i>According to the Centers for Disease Control and Prevention, supplementation of vitamin B<sub>12</sub> can be done orally with 1000 <span class="body">µ</span>g daily in patients with deficiency. In patients with active IBD, oral reabsorption of vitamin B<sub>12</sub> can be less effective, making subcutaneous or intramuscular administration (1000 <span class="body">µ</span>g/wk for 8 weeks, then monthly for life) better options.<sup>89</sup> <br/><br/>Patients with IBD managed with methotrexate should be screened carefully for folate deficiency. Methotrexate is a folate analog that sometimes is used for the treatment of IBD. Reversible competitive inhibition of dihydrofolate reductase can precipitate a systemic folic acid decrease.<sup>91</sup> Typically, oral folic acid (1 to 5 mg/d) is sufficient to treat folate deficiency, with the ESPEN recommending 5 mg once weekly 24 to 72 hours after methotrexate treatment or 1 mg daily for 5 days per week in patients with IBD.<sup>1</sup> Alternative formulations—IV, subcutaneous, or intramuscular—are available for patients who cannot tolerate oral intake.<sup>92</sup> </p> <h3>Final Thoughts</h3> <p>Dermatologists can be the first to observe the cutaneous manifestations of micronutrient deficiencies. Although the symptoms of each micronutrient deficiency discussed may overlap, attention to small clinical clues in patients with IBD can improve patient outcomes and quality of life. For example, koilonychia with glossitis and xerosis likely is due to iron deficiency, while zinc deficiency should be suspected in patients with scaly eczematous plaques in skin folds. A high level of suspicion for micronutrient deficiencies in patients with IBD should be followed by a complete patient history, review of systems, and thorough clinical examination. A thorough laboratory evaluation can pinpoint nutritional deficiencies in patients with IBD, keeping in mind that specific biomarkers such as ferritin and serum zinc also act as acute phase reactants and should be interpreted in this context. Co-management with gastroenterologists should be a priority in patients with IBD, as gaining control of inflammatory disease is crucial for the prevention of recurrent vitamin and micronutrient deficiencies in addition to long-term health in this population.</p> <h2>References </h2> <p class="reference"> 1. Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. <i>Clin Nutr</i>. 2023;42:352-379. doi:10.1016/j.clnu.2022.12.004<br/><br/> 2. Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammator y bowel disease. <i>J Hum Nutr Diet</i>. 2011;24:313-326. doi:10.1111/j.1365-277X.2011.01171.x<br/><br/> 3. Mentella MC, Scaldaferri F, Pizzoferrato M, et al. Nutrition, IBD and gut microbiota: a review. <i>Nutrients</i>. 2020;12:944. doi:10.3390/nu12040944<br/><br/> 4. Bonsack O, Caron B, Baumann C, et al. Food avoidance and fasting in patients with inflammatory bowel disease: experience from the Nancy IBD nutrition clinic. <i>United European Gastroenterol J</i>. 2023;11:361-370. doi:10.1002/ueg2.1238521 <br/><br/> 5. Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen Anti-Inflammatory Diet (GrAID). <i>Nutrients</i>. 2021;13:1067. doi:10.3390/nu13041067<br/><br/> 6. Hwang C, Issokson K, Giguere-Rich C, et al. Development and pilot testing of the inflammatory bowel disease nutrition care pathway. <i>Clin Gastroenterol Hepatol</i>. 2020;18:2645-2649.e4. doi:10.1016/j.cgh.2020.06.039 <br/><br/> 7. Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta-analysis. <i>Inflamm Bowel Dis</i>. 2014;20:936-945. doi:10.1097/01.MIB.0000442728.74340.fd<br/><br/> 8. Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. <i>Nat Rev Gastroenterol Hepatol</i>. 2010;7:599-610. doi:10.1038/nrgastro.2010.151<br/><br/> 9. Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. <i>StatPearls [Internet]</i>. Updated April 17, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448204/<br/><br/>10. Evstatiev R, Gasche C. Iron sensing and signalling. <i>Gut</i>. 2012;61:933-952. doi:10.1136/gut.2010.214312<br/><br/>11. Przybyszewska J, Zekanowska E. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. <i>Prz Gastroenterol</i>. 2014;9:208-213. doi:10.5114/pg.2014.45102<br/><br/>12. Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. <i>Haematologica</i>. 2010;95:175-178. doi:10.3324/haematol.2009.017046<br/><br/>13. Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. <i>World J Gastrointest Pathophysiol</i>. 2015;6:62-72. doi:10.4291/wjgp.v6.i3.62<br/><br/>14. Moiz B. Spoon nails: still seen in today’s world. <i>Clin Case Rep</i>. 2018;6:547-548. doi:10.1002/ccr3.1404<br/><br/>15. St Pierre SA, Vercellotti GM, Donovan JC, et al. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. <i>J Am Acad Dermatol</i>. 2010;63:1070-1076. doi:10.1016/j.jaad.2009.05.054<br/><br/>16. Chiang CP, Yu-Fong Chang J, Wang YP, et al. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in atrophic glossitis patients with or without microcytosis. <i>J Formos Med Assoc</i>. 2019;118:1401-1407. doi:10.1016/j.jfma.2019.06.004<br/><br/>17. Chiang CP, Chang JY, Wang YP, et al. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. <i>J Formos Med Assoc</i>. 2020;119:774-780. doi:10.1016/j.jfma.2019.04.015<br/><br/>18. Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. <i>J Eur Acad Dermatol Venereol</i>. 2016;30:1985-1991. doi:10.1111/jdv.13610<br/><br/>19. Guo HF, Tsai CL, Terajima M, et al. Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe<sup>2+</sup>-binding. <i>Nat Commun</i>. 2018;9:512. doi:10.1038/s41467-018-02859-z<br/><br/>20. Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. <i>Indian J Dermatol</i>. 2021;66:705. doi:10.4103/ijd.ijd_326_21<br/><br/>21. Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. <i>Science</i>. 2008;320:1088-1092. doi:10.1126/science.1157121<br/><br/>22. Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. <i>J Am Acad Dermatol</i>. 2019;80:466-477.e16. doi:10.1016/j.jaad.2018.07.013<br/><br/>23. Hashash JG, Elkins J, Lewis JD, et al. AGA Clinical Practice Update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review [published online January 23, 2024]. <i>Gastroenterology</i>. doi:10.1053/j.gastro.2023.11.303<br/><br/>24. Choudhuri S, Chowdhury IH, Saha A, et al. Acute monocyte pro- inflammatory response predicts higher positive to negative acute phase reactants ratio and severe hemostatic derangement in dengue fever. <i>Cytokine</i>. 2021;146:155644. doi:10.1016/j.cyto.2021.155644<br/><br/>25. Dignass AU, Gasche C, Bettenworth D, et al; European Crohn’s and Colitis Organisation. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. <i>J Crohn’s Colitis</i>. 2015;9:211-222. doi:10.1093/ecco-jcc/jju009 <br/><br/>26. Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. <i>Aliment Pharmacol Ther</i>. 2020;51:1087-1095. doi:10.1111/apt.15739<br/><br/>27. Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. <i>Am J Clin Nutr</i>. 2017;106(suppl 6):1606S-1614S. doi:10.3945/ajcn.117.155887<br/><br/>28. Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. <i>PLoS One</i>. 2015;10:e0117383. doi:10.1371/journal.pone.0117383<br/><br/>29. Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. <i>Gastroenterology</i>. 2011;141:846-853.e8532. doi:10.1053/j.gastro.2011.06.005<br/><br/>30. Zupo R, Sila A, Castellana F, et al. Prevalence of zinc deficiency in inflammatory bowel disease: a systematic review and meta-analysis. <i>Nutrients</i>. 2022;14:4052. doi:10.3390/nu14194052<br/><br/>31. Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. <i>Biometals</i>. 2022;35:187-213. doi:10.1007/s10534-022-00373-w<br/><br/>32. Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. <i>Nutrients</i>. 2020;12:762. doi:10.3390/nu12030762</p> <p class="reference">33. Ranaldi G, Ferruzza S, Canali R, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. <i>J Nutr Biochem</i>. 2013;24:967-976. doi:10.1016/j.jnutbio.2012.06.020<br/><br/>34. Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. <i>Arch Biochem Biophys</i>. 2016;611:113-119. doi:10.1016/j.abb.2016.06.003<br/><br/>35. Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. <i>Immunol Cell Biol</i>. 2006;84:28-37. doi:10.1111/j.1440-1711.2005.01391.x<br/><br/>36. Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. <i>Clin Dermatol</i>. 2010;28:669-685. doi:10.1016/j.clindermatol.2010.03.029<br/><br/>37. Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. <i>Am J Dermatopathol</i>. 1982;4:303-311.<br/><br/>38. Gammoh NZ, Rink L. Zinc in infection and inflammation. <i>Nutrients</i>. 2017;9:624. doi:10.3390/nu9060624<br/><br/>39. Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. <i>Proc Natl Acad Sci U S A</i>. 2005;102:6843-6848. doi:10.1073/pnas.0502257102<br/><br/>40. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. <i>Gut</i>. 2006;55:426-431. doi:10.1136/gut.2005.069476<br/><br/>41. Morisaku M, Ito K, Ogiso A, et al. Correlation between serum albumin and serum zinc in malignant lymphoma. <i>Fujita Med J</i>. 2022;8:59-64. doi:10.20407/fmj.2021-006<br/><br/>42. Falchuk KH. Effect of acute disease and ACTH on serum zinc proteins. <i>N Engl J Med</i>. 1977:296:1129-1134.<br/><br/>43. Naber TH, Baadenhuysen H, Jansen JB, et al. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. <i>Clin Chim Acta</i>. 1996;249:109-127. doi:10.1016/0009-8981(96)06281-x<br/><br/>44. Lowe D, Sanvictores T, Zubair M, et al. Alkaline phosphatase. <i>StatPearls [Internet]</i>. <span class="bk">Updated October 29, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459201/<br/><br/></span>45. Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. <i>Ann Nutr Metab</i>. 2013;62 suppl 1:19-29. doi:10.1159/000348261<br/><br/>46. Maxfield L, Shukla S, Crane JS. Zinc deficiency. <i>StatPearls [Internet]</i>. Updated June 28, 2023. Accessed March 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493231/ <br/><br/>47. Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. <i>Gastroenterol Clin North Am</i>. 2017;46:797-808. doi:10.1016/j.gtc.2017.08.011<br/><br/>48. Caviezel D, Maissen S, Niess JH, et al. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. <i>Inflamm Intest Dis</i>. 2018;2:200-210. doi:10.1159/000489010<br/><br/>49. Jasielska M, Grzybowska-Chlebowczyk U. Hypocalcemia and vitamin D deficiency in children with inflammatory bowel diseases and lactose intolerance. <i>Nutrients</i>. 2021;13:2583. doi:10.3390/nu13082583<br/><br/>50. Vernia F, Valvano M, Longo S, et al. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. <i>Nutrients</i>. 2022;14:269. doi:10.3390/nu14020269 <br/><br/>51. Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. <i>Curr Rheumatol Rep</i>. 2008;10:110-117. doi:10.1007/s11926-008-0020-y<br/><br/>52. Domazetovic V, Iantomasi T, Bonanomi AG, et al. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. <i>Int J Colorectal Dis</i>. 2020;35:1231-1242. doi:10.1007/s00384-020-03576-0<br/><br/>53. Gubatan J, Chou ND, Nielsen OH, et al. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. <i>Aliment Pharmacol Ther</i>. 2019;50:1146-1158. doi:10.1111/apt.15506<br/><br/>54. Fakhoury HMA, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. <i>J Steroid Biochem Mol Biol</i>. 2020;200:105663. doi:10.1016/j.jsbmb.2020.105663<br/><br/>55. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. <i>Science</i>. 2006;311:1770-1773. doi:10.1126/science.1123933<br/><br/>56. Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. <i>J Adv Res</i>. 2015;6:793-804. doi:10.1016/j.jare.2014.01.011<br/><br/>57. Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. <i>Immunol Allergy Clin North Am</i>. 2010;30:397-409.<br/><br/>58. Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. <i>Clin Exp Dermatol</i>. 2018;43:529-535.<br/><br/>59. Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. <i>Nat Clin Pract Rheumatol</i>. 2008;4:404-412.<br/><br/>60. Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. <i>Lancet Diabetes Endocrinol</i>. 2014;2:76-89. doi:10.1016/S2213-8587(13)70165-7<br/><br/>61. Schafer AL, Shoback DM. Hypocalcemia: diagnosis and treatment. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. <i>Endotext [Internet]</i>. Updated January 3, 2016. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK279022/<br/><br/>62. Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. <i>J Crohns Colitis</i>. 2017;11:649-670. doi:10.1093/ecco-jcc/jjx008<br/><br/>63. Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. <i>Eur J Clin Nutr</i>. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y<br/><br/>64. Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. <i>J Clin Endocrinol Metab</i>. 2016;101:394-415. doi:10.1210/jc.2015-2175</p> <p class="reference">65. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. <i>Dietary Reference Intakes for Calcium and Vitamin D</i>. National Academies Press (US); 2011.<br/><br/>66. Yeaman F, Nguyen A, Abasszade J, et al. Assessing vitamin D as a biomarker in inflammatory bowel disease. <i>JGH Open</i>. 2023;7:953-958. doi:10.1002/jgh3.13010<br/><br/>67. Vernia P, Loizos P, Di Giuseppantonio I, et al S. Dietary calcium intake in patients with inflammatory bowel disease. <i>J Crohns Colitis</i>. 2014;8:312-317. doi:10.1016/j.crohns.2013.09.008<br/><br/>68. Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. <i>BMJ</i>. 2008;336:1298-1302. doi:10.1136/bmj.39582.589433.BE<br/><br/>69. Kenny CM, Murphy CE, Boyce DS, et al. Things we do for no reason™: calculating a “corrected calcium” level. <i>J Hosp Med</i>. 2021;16:499-501. doi:10.12788/jhm.3619<br/><br/>70. Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. <i>Clin Nutr. </i>2018;37:1375-1382. doi:10.1016/j.clnu.2017.06.011<br/><br/>71. Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. <i>United European Gastroenterol J.</i> 2015;3:294-302. doi:10.1177/2050640615572176<br/><br/>72. Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. <i>JPEN J Parenter Enteral Nutr</i>. 2007;31:311-319. doi:10.1177/0148607107031004311<br/><br/>73. Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency.<i> J Am Acad Dermatol</i>. 1986;15:1263-1274. doi:10.1016/s0190-9622(86)70301-0<br/><br/>74. Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. <i>Cleve Clin J Med</i>. 2016;83:731-739. doi:10.3949/ccjm.83a.15061<br/><br/>75. Elgharably N, Al Abadie M, Al Abadie M, et al. Vitamin B group levels and supplementations in dermatology. <i>Dermatol Reports</i>. 2022;15:9511. doi:10.4081/dr.2022.9511<br/><br/>76. Hołubiec P, Leon´czyk M, Staszewski F, et al. Pathophysiology and clinical management of pellagra—a review. <i>Folia Med Cracov</i>. 2021;61:125-137. doi:10.24425/fmc.2021.138956 <br/><br/>77. Ink SL, Henderson LM. Vitamin B6 metabolism. <i>Annu Rev Nutr</i>. 1984;4:455-470. doi:10.1146/annurev.nu.04.070184.002323<br/><br/>78. Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 deficiency. <i>StatPearls [Internet]</i>. Updated August 8, 2023. Accessed March 25, 2024. <a href="https://www.ncbi.nlm.nih.gov/books/NBK470579/">https://www.ncbi.nlm.nih.gov/books/NBK470579/</a>. <br/><br/>79. Vasilaki AT, McMillan DC, Kinsella J, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. <i>Am J Clin Nutr</i>. 2008;88:140-146. doi:10.1093/ajcn/88.1.140<br/><br/>80. Chiang EP, Bagley PJ, Selhub J, et al. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. <i>Am J Med</i>. 2003;114:283-287. doi:10.1016/s0002-9343(02)01528-0<br/><br/>81. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. <i>J Crohns Colitis.</i> 2019;13:144-164. doi:10.1093/ecco-jcc/jjy113<br/><br/>82. Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. <i>Nutr Hosp</i>. 2007;22:7-24.<br/><br/>83. Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. <i>J Nutr Biochem</i>. 2013;24:2138-2143. doi:10.1016/j.jnutbio.2013.08.005<br/><br/>84. Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. <i>Nutrients</i>. 2017;9:382. doi:10.3390/nu9040382<br/><br/>85. Brescoll J, Daveluy S. A review of vitamin B<sub>12</sub> in dermatology. <i>Am J Clin Dermatol</i>. 2015;16:27-33. doi:10.1007/s40257-014-0107-3<br/><br/>86. DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. <i>Nutr Clin Pract</i>. 2019;34:490-503. doi:10.1002/ncp.10321<br/><br/>87. Mori K, Ando I, Kukita A. Generalized hyperpigmentation of the skin due to vitamin B<sub>12</sub> deficiency. <i>J Dermatol</i>. 2001;28:282-285. doi:10.1111/j.1346-8138.2001.tb00134.x<br/><br/>88. Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. <i>Am J Clin Nutr</i>. 2011;94:666S-672S. doi:10.3945/ajcn.110.009613<br/><br/>89. NIH Office of Dietary Supplements. Vitamin B<sub>12</sub>: fact sheet for health professionals. Updated February 27, 2024. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/<br/><br/>90. NIH Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 20, 2023. Accessed March 19, 2024. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.<br/><br/>91. Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. <i>Dig Liver Dis</i>. 2012;44:123-127. doi:10.1016/j.dld.2011.09.015<br/><br/>92. Khan KM, Jialal I. Folic acid deficiency. <i>StatPearls [Internet]</i>. Updated June 26, 2023. Accessed March 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535377/</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>bio</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="disclosure">From the University of Wisconsin School of Medicine and Public Health, Madison. Todd A. Le and Dr. Shields are from the Department of Dermatology, and Dr. Saha is from the Department of Medicine, Division of Gastroenterology and Hepatology.</p> <p class="disclosure">Todd A. Le and Dr. Shields report no conflict of interest. Dr. Saha is part-owner of BrainSync Rehabilitation, Inc.Correspondence: Bridget E. Shields, MD, Department of Dermatology, University of Wisconsin, 1 S Park St, Madison, WI 53715 (bshields@dermatology.wisc.edu).<br/><br/><em>Cutis.</em> 2024 April;113(4):159-166. doi:10.12788/cutis.0993</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>in</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="insidehead">Practice <strong>Points</strong></p> <ul class="insidebody"> <li>Patients with inflammatory bowel disease (IBD) are at increased risk for vitamin and nutrient deficiencies that may be identified first through cutaneous manifestations.</li> <li>Because active inflammation in IBD may skew routine laboratory values used for screening of micronutrient deficiencies, be cautious when interpreting these values. </li> <li>Patients taking systemic therapies for IBD such as corticosteroids and methotrexate are at higher risk for nutritional deficiencies. </li> </ul> </itemContent> </newsItem> </itemSet></root>
Practice Points
- Patients with inflammatory bowel disease (IBD) are at increased risk for vitamin and nutrient deficiencies that may be identified first through cutaneous manifestations.
- Because active inflammation in IBD may skew routine laboratory values used for screening of micronutrient deficiencies, be cautious when interpreting these values.
- Patients taking systemic therapies for IBD such as corticosteroids and methotrexate are at higher risk for nutritional deficiencies.
Recurrent Aphthous Stomatitis: Clinical Experience From a University Hospital in Brazil
To the Editor:
Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,1,2 round ulcerations of variable sizes with a tendency for recurrence, most commonly located in nonkeratinized areas of the oral mucosa. Pathergy commonly is observed.3 Although many authors consider the terms RAS andaphtha to be synonymous,4,5 differentiating the clinical lesion (aphthous ulceration) from the disease (aphtha or RAS) can be useful, as several other diseases can at times manifest with similar ulcers (called aphthoid lesions), such as pemphigus vulgaris, mucous membrane pemphigoid, and erythema multiforme.6
It is estimated that approximately 20% of individuals worldwide have at least one episode of aphtha during their lifetime,7 and it is considered the most common disease of the oral mucosa.8,9 However, only patients presenting with severe acute outbreaks or frequent relapses typically seek medical treatment. Clinically, aphthous ulcers are classified as aphtha minor (small number of small lesions), aphtha major (large deep lesions that also can affect the minor salivary glands with intense necrosis, difficulty in healing, and mucosal scarring), and aphtha herpetiformis (innumerous tiny lesions that reappear in recurring outbreaks).1-3 The term complex aphthosis was introduced in 198510 and is defined as recurrent oral and genital aphthous ulcerations or recurring multiple oral aphthous ulcers in the absence of systemic manifestations or Behçet disease11,12; however, complex aphthosis also has been reported as frequent episodes of ulcerations that may be associated with systemic diseases including Behçet disease.13,14
Currently, RAS is considered an immunologically mediated alteration in cutaneous mucosal reactivity with a multifactorial systemic cause. Underlying conditions such as Behçet disease, inflammatory bowel disease (IBD), iatrogenic immunosuppression (eg, following solid organ transplantation), AIDS, and cyclic neutropenia may or may not be detected.11-13
Our retrospective study explored the systemic nature of RAS. We reviewed patient records to evaluate underlying systemic conditions associated with the diagnosis of RAS and the use of oral medications in managing the disease. Medical records from the Department of Dermatology of the University of São Paulo, Brazil, from 2003 to 2017 were reviewed to identify patients with a diagnosis of RAS. Clinical classification of RAS—minor, major, or herpetiform—as well as the presence of aphthous lesions in other locations and the presence of other associated inflammatory cutaneous manifestations also were noted. Associated systemic diseases and treatments for RAS were recorded. Patients for whom the diagnosis of RAS was changed during follow-up were excluded. Because this was a retrospective analysis of medical records and without any patient risk, informed consent was not needed.
Medical records for 125 patients were reviewed; 63 were male (50.4%), and 62 were female (49.6%). The age at onset of symptoms, which ranged from a few months after birth to 74 years, was reported in only 92 (73.6%) patient medical records. Of these, 30 (32.6%) reported onset before 20 years of age, 39 (42.4%) between 20 and 39 years, 17 (18.5%) between 40 and 59 years, and 6 (6.5%) at 60 years or older. Morphologically, 72 (57.6%) had minor, 42 (33.6%) had major, and 11 (8.8%) had herpetiform aphthous ulcers. None of the patients presented with sporadic lesions; the disease was long-standing and persistent in all cases (complex aphthosis).
Regarding the location of the ulcers, 92 (73.6%) patients had lesions on the oral mucosa only. Some patients had lesions in more than one site in addition to the oral mucosa: 32 (25.6%) had aphthae in the genital/groin region and 4 (3.2%) presented with perianal/anal aphthae. Nineteen patients (19.2%) presented other cutaneous manifestations in addition to aphthae: 11 (45.8%) had folliculitis/pseudofolliculitis, and 8 (33.3%) had erythema nodosum (EN). Eight patients (33.3%) presented with uveitis, and 6 (25%) presented with concomitant arthralgia/arthritis. Fifty-four patients (43.2%) had confirmed or suspected associated disease: Behçet disease (21 [38.9%]), IBD (10 [18.5%]), solid organ transplantation (7 [13.0%])(kidney, 4 [57.1%]; heart, 2 [28.6%]; liver, 1 [14.3%]), HIV infection (6 [11.1%]), lymphoma (1 [1.9%]), aplastic anemia (1 [1.9%]), or myelodysplastic syndrome (1 [1.9%]). Ten patients (18.5%) presented with other diseases under investigation (eg, unidentified rheumatologic disease, unexplained neutropenia, undiagnosed immunodeficiencies, autoinflammatory syndromes, possible cyclic neutropenia).
Biopsies of the oral mucosa were performed in 31 patients. Histopathologic findings will be discussed in a future publication (unpublished data).
Five patients (4.0%) were lost to follow-up and did not receive treatment; 10 (8.0%) received only topical treatment (analgesics and/or corticosteroids). All 9 (7.2%) patients undergoing intralesional corticosteroid injections also were on a systemic treatment. One hundred ten (88.0%) patients were treated systemically—with colchicine (84/110 [76.4%]), thalidomide (43/110 [39.1%]), small pulses of oral corticosteroids (26 [23.6%]), dapsone (12/110 [10.9%]), or pentoxifylline (3 [2.7%]). Furthermore, in patients with associated diseases, treatment of the underlying condition was conducted when available, and follow-up was carried out in conjunction with the appropriate specialists. For treatment of the associated disease, patients received other medications such as methotrexate, azathioprine, cyclophosphamide, intravenous corticosteroid pulse, and immunobiologics.
The prevalence of RAS between sexes in our study population was similar (50.4% male; 49.6% female). Results from prior studies have been mixed; some reported a higher prevalence in females,15-18 while others found no predilection for sex among patients diagnosed with RAS.19,20 In our analysis, 75% of patients experienced symptoms of RAS before 40 years of age; in prior studies, up to 56% of patients experienced symptoms between the ages of 20 and 40 years.21,22
In our study, 26.4% of patients had extraoral aphthae. Genital lesions have been described as infrequent,23 and lesions manifesting in other mucous membranes or on the skin are rare.24 A study reported genital involvement in 8% to 13% of patients with oral aphtha.25 We observed genital involvement in 25.6% of patients. Likewise, this higher value may be due to our study population of patients referred to our university hospital. In our study, 19.2% of patients presented with other inflammatory manifestations in addition to aphthous ulcerations (eg, folliculitis, EN, uveitis, arthritis). As dermatologists in a tertiary reference hospital, we actively look for such associations in every aphtha patient, which may not be the case in many nondermatologic oral care services.
In our study population, 43.2% of patients were diagnosed with or were under investigation for systemic diseases known to be associated with RAS. We found associations with Behçet disease most frequently, followed by IBD,26 solid organ transplantation, and HIV. In this group of patients, the respective systemic disease was active or poorly controlled. In transplant recipients, aphtha major was the most common type, similar to other studies.27 We observed no notable difference in the clinical picture of the oral ulcers in patients with a well-established systemic disease vs those without.
Most of our cases did not present findings other than aphtha, indicating that the intrinsic defect that predisposes to RAS is always systemic. Even mild and sporadic cases may be attributable to a systemic disorder of cutaneous-mucosal reactivity. The predisposition to RAS never originates in the oral cavity, hence the confusion caused and the uselessness of studies that relate aphthae to factors such as local food allergies, pH changes, or local infection with microorganisms.5,28 The disease course (reducing the frequency of lesion appearance and accelerating the healing of extensive lesions) is only modified with systemic treatment, with local measures proving to be only moderately useful to relieve pain. We believe that RAS can in many ways be compared to EN and pyoderma gangrenosum (PG): some systemic conditions that predispose patients to EN and PG also may predispose them to RAS (eg, IBD, hematologic disorders). Similar to RAS, many cases of EN and PG are idiopathic. In addition, pathergy also occurs in PG.11,13
We were unable to observe or establish any predictive clinical element that could indicate a better or worse response to the prescribed treatments, which also has been noted by other authors.3,4 Treatment of RAS is empiric, generally starting with drugs that are easier to prescribe and with fewer adverse effects, then progressing to more complex drugs when a good response is not obtained. Colchicine was the most commonly prescribed medication (76.4% [84/110]). It has been proposed by several authors3,4 as a first-line systemic medication for the treatment of recurrent aphthae, as it has been shown to be effective and safe. The dosage ranged from 0.5 mg twice daily to 0.5 mg 4 times daily. Dapsone is an established drug for aphtha29,30 and was used in 12 of our patients. The dosage used in our patients ranged from 50 to 100 mg/d. Adverse effects such as hemolytic anemia frequently are seen, and one of the patients in our study developed DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome in response to dapsone. In 7 cases, colchicine and dapsone were used together, which is believed to potentiate the therapeutic effects. This combination may be useful in patients for whom thalidomide cannot be used or those who have not improved with monotherapy.29 Thalidomide is considered one of the most effective drugs for RAS.30,31 Forty-three patients in our analysis were treated with thalidomide,usually as a first choice. The dosage ranged from 100 to 200 mg/d. It was mainly chosen in disabling pediatric cases, adult men with aphthous major, and women with no risk for pregnancy. Due to its potential adverse effects, thalidomide has been recommended when there is no response with other medications that are dose dependent; severe adverse effects such as thromboembolism and peripheral neuropathy are rare.31 Oral corticosteroids were used in 26 patients, aiming at rapid improvement in very symptomatic cases; however, due to the potential for long-term adverse effects, in all cases they were prescribed in combination with another medication that was maintained after the corticosteroid was discontinued.
We highlight the systemic nature of RAS as well as its frequent association with systemic diseases and other correlated manifestations (pustules, EN, arthralgia). We also emphasize the importance of using oral medications to adequately control the disease and do not recommend topical medications aimed at treating local causes. Dermatologists should be consulted in managing severe cases of RAS.
- Buño IJ, Huff JC, Weston WL, et al. Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch Dermatol. 1998;134:827-831.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728- 732.
- Natah SS, Konttinen YT, Enattah NS, et al. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221-234.
- Zunt SL. Recurrent aphthous stomatitis. Dermatol Clin. 2003;21:33-39.
- Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. number VI. recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21.
- Chams-Davatchi C, Shizarpour M, Davatchi F, et al. Comparison of oral aphthae in Behçet’s disease and idiopathic recurrent aphthous stomatitis. Adv Exp Med Biol. 2003;528:317-320.
- Schemel-Suárez M, López-López J, Chimenos-Küstner E. Oral ulcers: differential diagnosis and treatment [in Spanish]. Med Clin (Barc). 2015;145:499-503.
- S´lebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz). 2014;62:205-215.
- Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10:26-36.
- Jorizzo JL, Taylor RS, Schmalstieg FC, et al. Complex aphthosis: a forme fruste of Behçet’s syndrome? J Am Acad Dermatol. 1985;13:80-84.
- McCarty MA, Garton RA, Jorizzo JL. Complex aphthosis and Behçet’s disease. Dermatol Clin. 2003;21:41-48.
- Bulur I, Melrem O. Behçet disease: new aspects. Clin Dermatol. 2017;35:421-434.
- Cui RZ, Rogers RS 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34:475-481.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728-732.
- Ship II. Epidemiologic aspects of recurrent aphthous ulcerations. Oral Surg Oral Med Oral Pathol. 1972;33:400-406.
- Ship JA. Recurrent aphthous stomatitis. an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:141-147.
- Wilhelmsen NS, Weber R, Monteiro F, et al. Correlation between histocompatibility antigens and recurrent aphthous stomatitis in the Brazilian population. Braz J Otorhinolaryngol. 2009;75:426-431.
- S´lebioda Z, Dorocka-Bobkowska B. Systemic and environmental risk factors for recurrent aphthous stomatitis in a Polish cohort of patients. Postepy Dermatol Alergol. 2019;36:196-201.
- Ship JA, Chavez EM, Doerr PA, et al. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95-112.
- Brocklehurst P, Tickle M, Glenny AM, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database Syst Rev. 2012;12:CD005411.
- Belenguer-Guallar I, Jiménez-Soriano Y, Ariadna Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis. a literature review. J Clin Exp Dent. 2014;6:E168-E174.
- Bagán JV, Sanchis JM, Milián MA, et al. Recurrent aphthous stomatitis. a study of the clinical characteristics of lesions in 93 cases. J Oral Pathol Med. 1991;20:395-397.
- Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19:195-204.
- Scully C, Porter S. Recurrent aphthous stomatitis: current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18:21-27
- Chapel TA. Origins of penile ulcerations. Arch Androl. 1979; 3: 351-357.
- Lourenço SV, Hussein TP, Bologna SB, et al. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204-207.
- Nico MM, Brito AE, Martins LE, et al. Oral ulcers in an immunosuppressed 5-year-old boy. Clin Exp Dermatol. 2008;33:367-368.
- Trakji B, Baroudi K, Kharma Y. The effect of dietary habits on the development of the recurrent aphthous stomatitis. Niger Med J. 2012;53:9-11.
- Lynde CB, Bruce AJ, Rogers RS 3rd. Successful treatment of complex aphthosis with colchicine and dapsone. Arch Dermatol. 2009;145:273-276.
- Letsinger JA, McCarty MA, Jorizzo JL. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J Am Acad Dermatol. 2005(3 pt 1);52:500-508.
- Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. Medicine (Baltimore). 2010;89:176-182.
To the Editor:
Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,1,2 round ulcerations of variable sizes with a tendency for recurrence, most commonly located in nonkeratinized areas of the oral mucosa. Pathergy commonly is observed.3 Although many authors consider the terms RAS andaphtha to be synonymous,4,5 differentiating the clinical lesion (aphthous ulceration) from the disease (aphtha or RAS) can be useful, as several other diseases can at times manifest with similar ulcers (called aphthoid lesions), such as pemphigus vulgaris, mucous membrane pemphigoid, and erythema multiforme.6
It is estimated that approximately 20% of individuals worldwide have at least one episode of aphtha during their lifetime,7 and it is considered the most common disease of the oral mucosa.8,9 However, only patients presenting with severe acute outbreaks or frequent relapses typically seek medical treatment. Clinically, aphthous ulcers are classified as aphtha minor (small number of small lesions), aphtha major (large deep lesions that also can affect the minor salivary glands with intense necrosis, difficulty in healing, and mucosal scarring), and aphtha herpetiformis (innumerous tiny lesions that reappear in recurring outbreaks).1-3 The term complex aphthosis was introduced in 198510 and is defined as recurrent oral and genital aphthous ulcerations or recurring multiple oral aphthous ulcers in the absence of systemic manifestations or Behçet disease11,12; however, complex aphthosis also has been reported as frequent episodes of ulcerations that may be associated with systemic diseases including Behçet disease.13,14
Currently, RAS is considered an immunologically mediated alteration in cutaneous mucosal reactivity with a multifactorial systemic cause. Underlying conditions such as Behçet disease, inflammatory bowel disease (IBD), iatrogenic immunosuppression (eg, following solid organ transplantation), AIDS, and cyclic neutropenia may or may not be detected.11-13
Our retrospective study explored the systemic nature of RAS. We reviewed patient records to evaluate underlying systemic conditions associated with the diagnosis of RAS and the use of oral medications in managing the disease. Medical records from the Department of Dermatology of the University of São Paulo, Brazil, from 2003 to 2017 were reviewed to identify patients with a diagnosis of RAS. Clinical classification of RAS—minor, major, or herpetiform—as well as the presence of aphthous lesions in other locations and the presence of other associated inflammatory cutaneous manifestations also were noted. Associated systemic diseases and treatments for RAS were recorded. Patients for whom the diagnosis of RAS was changed during follow-up were excluded. Because this was a retrospective analysis of medical records and without any patient risk, informed consent was not needed.
Medical records for 125 patients were reviewed; 63 were male (50.4%), and 62 were female (49.6%). The age at onset of symptoms, which ranged from a few months after birth to 74 years, was reported in only 92 (73.6%) patient medical records. Of these, 30 (32.6%) reported onset before 20 years of age, 39 (42.4%) between 20 and 39 years, 17 (18.5%) between 40 and 59 years, and 6 (6.5%) at 60 years or older. Morphologically, 72 (57.6%) had minor, 42 (33.6%) had major, and 11 (8.8%) had herpetiform aphthous ulcers. None of the patients presented with sporadic lesions; the disease was long-standing and persistent in all cases (complex aphthosis).
Regarding the location of the ulcers, 92 (73.6%) patients had lesions on the oral mucosa only. Some patients had lesions in more than one site in addition to the oral mucosa: 32 (25.6%) had aphthae in the genital/groin region and 4 (3.2%) presented with perianal/anal aphthae. Nineteen patients (19.2%) presented other cutaneous manifestations in addition to aphthae: 11 (45.8%) had folliculitis/pseudofolliculitis, and 8 (33.3%) had erythema nodosum (EN). Eight patients (33.3%) presented with uveitis, and 6 (25%) presented with concomitant arthralgia/arthritis. Fifty-four patients (43.2%) had confirmed or suspected associated disease: Behçet disease (21 [38.9%]), IBD (10 [18.5%]), solid organ transplantation (7 [13.0%])(kidney, 4 [57.1%]; heart, 2 [28.6%]; liver, 1 [14.3%]), HIV infection (6 [11.1%]), lymphoma (1 [1.9%]), aplastic anemia (1 [1.9%]), or myelodysplastic syndrome (1 [1.9%]). Ten patients (18.5%) presented with other diseases under investigation (eg, unidentified rheumatologic disease, unexplained neutropenia, undiagnosed immunodeficiencies, autoinflammatory syndromes, possible cyclic neutropenia).
Biopsies of the oral mucosa were performed in 31 patients. Histopathologic findings will be discussed in a future publication (unpublished data).
Five patients (4.0%) were lost to follow-up and did not receive treatment; 10 (8.0%) received only topical treatment (analgesics and/or corticosteroids). All 9 (7.2%) patients undergoing intralesional corticosteroid injections also were on a systemic treatment. One hundred ten (88.0%) patients were treated systemically—with colchicine (84/110 [76.4%]), thalidomide (43/110 [39.1%]), small pulses of oral corticosteroids (26 [23.6%]), dapsone (12/110 [10.9%]), or pentoxifylline (3 [2.7%]). Furthermore, in patients with associated diseases, treatment of the underlying condition was conducted when available, and follow-up was carried out in conjunction with the appropriate specialists. For treatment of the associated disease, patients received other medications such as methotrexate, azathioprine, cyclophosphamide, intravenous corticosteroid pulse, and immunobiologics.
The prevalence of RAS between sexes in our study population was similar (50.4% male; 49.6% female). Results from prior studies have been mixed; some reported a higher prevalence in females,15-18 while others found no predilection for sex among patients diagnosed with RAS.19,20 In our analysis, 75% of patients experienced symptoms of RAS before 40 years of age; in prior studies, up to 56% of patients experienced symptoms between the ages of 20 and 40 years.21,22
In our study, 26.4% of patients had extraoral aphthae. Genital lesions have been described as infrequent,23 and lesions manifesting in other mucous membranes or on the skin are rare.24 A study reported genital involvement in 8% to 13% of patients with oral aphtha.25 We observed genital involvement in 25.6% of patients. Likewise, this higher value may be due to our study population of patients referred to our university hospital. In our study, 19.2% of patients presented with other inflammatory manifestations in addition to aphthous ulcerations (eg, folliculitis, EN, uveitis, arthritis). As dermatologists in a tertiary reference hospital, we actively look for such associations in every aphtha patient, which may not be the case in many nondermatologic oral care services.
In our study population, 43.2% of patients were diagnosed with or were under investigation for systemic diseases known to be associated with RAS. We found associations with Behçet disease most frequently, followed by IBD,26 solid organ transplantation, and HIV. In this group of patients, the respective systemic disease was active or poorly controlled. In transplant recipients, aphtha major was the most common type, similar to other studies.27 We observed no notable difference in the clinical picture of the oral ulcers in patients with a well-established systemic disease vs those without.
Most of our cases did not present findings other than aphtha, indicating that the intrinsic defect that predisposes to RAS is always systemic. Even mild and sporadic cases may be attributable to a systemic disorder of cutaneous-mucosal reactivity. The predisposition to RAS never originates in the oral cavity, hence the confusion caused and the uselessness of studies that relate aphthae to factors such as local food allergies, pH changes, or local infection with microorganisms.5,28 The disease course (reducing the frequency of lesion appearance and accelerating the healing of extensive lesions) is only modified with systemic treatment, with local measures proving to be only moderately useful to relieve pain. We believe that RAS can in many ways be compared to EN and pyoderma gangrenosum (PG): some systemic conditions that predispose patients to EN and PG also may predispose them to RAS (eg, IBD, hematologic disorders). Similar to RAS, many cases of EN and PG are idiopathic. In addition, pathergy also occurs in PG.11,13
We were unable to observe or establish any predictive clinical element that could indicate a better or worse response to the prescribed treatments, which also has been noted by other authors.3,4 Treatment of RAS is empiric, generally starting with drugs that are easier to prescribe and with fewer adverse effects, then progressing to more complex drugs when a good response is not obtained. Colchicine was the most commonly prescribed medication (76.4% [84/110]). It has been proposed by several authors3,4 as a first-line systemic medication for the treatment of recurrent aphthae, as it has been shown to be effective and safe. The dosage ranged from 0.5 mg twice daily to 0.5 mg 4 times daily. Dapsone is an established drug for aphtha29,30 and was used in 12 of our patients. The dosage used in our patients ranged from 50 to 100 mg/d. Adverse effects such as hemolytic anemia frequently are seen, and one of the patients in our study developed DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome in response to dapsone. In 7 cases, colchicine and dapsone were used together, which is believed to potentiate the therapeutic effects. This combination may be useful in patients for whom thalidomide cannot be used or those who have not improved with monotherapy.29 Thalidomide is considered one of the most effective drugs for RAS.30,31 Forty-three patients in our analysis were treated with thalidomide,usually as a first choice. The dosage ranged from 100 to 200 mg/d. It was mainly chosen in disabling pediatric cases, adult men with aphthous major, and women with no risk for pregnancy. Due to its potential adverse effects, thalidomide has been recommended when there is no response with other medications that are dose dependent; severe adverse effects such as thromboembolism and peripheral neuropathy are rare.31 Oral corticosteroids were used in 26 patients, aiming at rapid improvement in very symptomatic cases; however, due to the potential for long-term adverse effects, in all cases they were prescribed in combination with another medication that was maintained after the corticosteroid was discontinued.
We highlight the systemic nature of RAS as well as its frequent association with systemic diseases and other correlated manifestations (pustules, EN, arthralgia). We also emphasize the importance of using oral medications to adequately control the disease and do not recommend topical medications aimed at treating local causes. Dermatologists should be consulted in managing severe cases of RAS.
To the Editor:
Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,1,2 round ulcerations of variable sizes with a tendency for recurrence, most commonly located in nonkeratinized areas of the oral mucosa. Pathergy commonly is observed.3 Although many authors consider the terms RAS andaphtha to be synonymous,4,5 differentiating the clinical lesion (aphthous ulceration) from the disease (aphtha or RAS) can be useful, as several other diseases can at times manifest with similar ulcers (called aphthoid lesions), such as pemphigus vulgaris, mucous membrane pemphigoid, and erythema multiforme.6
It is estimated that approximately 20% of individuals worldwide have at least one episode of aphtha during their lifetime,7 and it is considered the most common disease of the oral mucosa.8,9 However, only patients presenting with severe acute outbreaks or frequent relapses typically seek medical treatment. Clinically, aphthous ulcers are classified as aphtha minor (small number of small lesions), aphtha major (large deep lesions that also can affect the minor salivary glands with intense necrosis, difficulty in healing, and mucosal scarring), and aphtha herpetiformis (innumerous tiny lesions that reappear in recurring outbreaks).1-3 The term complex aphthosis was introduced in 198510 and is defined as recurrent oral and genital aphthous ulcerations or recurring multiple oral aphthous ulcers in the absence of systemic manifestations or Behçet disease11,12; however, complex aphthosis also has been reported as frequent episodes of ulcerations that may be associated with systemic diseases including Behçet disease.13,14
Currently, RAS is considered an immunologically mediated alteration in cutaneous mucosal reactivity with a multifactorial systemic cause. Underlying conditions such as Behçet disease, inflammatory bowel disease (IBD), iatrogenic immunosuppression (eg, following solid organ transplantation), AIDS, and cyclic neutropenia may or may not be detected.11-13
Our retrospective study explored the systemic nature of RAS. We reviewed patient records to evaluate underlying systemic conditions associated with the diagnosis of RAS and the use of oral medications in managing the disease. Medical records from the Department of Dermatology of the University of São Paulo, Brazil, from 2003 to 2017 were reviewed to identify patients with a diagnosis of RAS. Clinical classification of RAS—minor, major, or herpetiform—as well as the presence of aphthous lesions in other locations and the presence of other associated inflammatory cutaneous manifestations also were noted. Associated systemic diseases and treatments for RAS were recorded. Patients for whom the diagnosis of RAS was changed during follow-up were excluded. Because this was a retrospective analysis of medical records and without any patient risk, informed consent was not needed.
Medical records for 125 patients were reviewed; 63 were male (50.4%), and 62 were female (49.6%). The age at onset of symptoms, which ranged from a few months after birth to 74 years, was reported in only 92 (73.6%) patient medical records. Of these, 30 (32.6%) reported onset before 20 years of age, 39 (42.4%) between 20 and 39 years, 17 (18.5%) between 40 and 59 years, and 6 (6.5%) at 60 years or older. Morphologically, 72 (57.6%) had minor, 42 (33.6%) had major, and 11 (8.8%) had herpetiform aphthous ulcers. None of the patients presented with sporadic lesions; the disease was long-standing and persistent in all cases (complex aphthosis).
Regarding the location of the ulcers, 92 (73.6%) patients had lesions on the oral mucosa only. Some patients had lesions in more than one site in addition to the oral mucosa: 32 (25.6%) had aphthae in the genital/groin region and 4 (3.2%) presented with perianal/anal aphthae. Nineteen patients (19.2%) presented other cutaneous manifestations in addition to aphthae: 11 (45.8%) had folliculitis/pseudofolliculitis, and 8 (33.3%) had erythema nodosum (EN). Eight patients (33.3%) presented with uveitis, and 6 (25%) presented with concomitant arthralgia/arthritis. Fifty-four patients (43.2%) had confirmed or suspected associated disease: Behçet disease (21 [38.9%]), IBD (10 [18.5%]), solid organ transplantation (7 [13.0%])(kidney, 4 [57.1%]; heart, 2 [28.6%]; liver, 1 [14.3%]), HIV infection (6 [11.1%]), lymphoma (1 [1.9%]), aplastic anemia (1 [1.9%]), or myelodysplastic syndrome (1 [1.9%]). Ten patients (18.5%) presented with other diseases under investigation (eg, unidentified rheumatologic disease, unexplained neutropenia, undiagnosed immunodeficiencies, autoinflammatory syndromes, possible cyclic neutropenia).
Biopsies of the oral mucosa were performed in 31 patients. Histopathologic findings will be discussed in a future publication (unpublished data).
Five patients (4.0%) were lost to follow-up and did not receive treatment; 10 (8.0%) received only topical treatment (analgesics and/or corticosteroids). All 9 (7.2%) patients undergoing intralesional corticosteroid injections also were on a systemic treatment. One hundred ten (88.0%) patients were treated systemically—with colchicine (84/110 [76.4%]), thalidomide (43/110 [39.1%]), small pulses of oral corticosteroids (26 [23.6%]), dapsone (12/110 [10.9%]), or pentoxifylline (3 [2.7%]). Furthermore, in patients with associated diseases, treatment of the underlying condition was conducted when available, and follow-up was carried out in conjunction with the appropriate specialists. For treatment of the associated disease, patients received other medications such as methotrexate, azathioprine, cyclophosphamide, intravenous corticosteroid pulse, and immunobiologics.
The prevalence of RAS between sexes in our study population was similar (50.4% male; 49.6% female). Results from prior studies have been mixed; some reported a higher prevalence in females,15-18 while others found no predilection for sex among patients diagnosed with RAS.19,20 In our analysis, 75% of patients experienced symptoms of RAS before 40 years of age; in prior studies, up to 56% of patients experienced symptoms between the ages of 20 and 40 years.21,22
In our study, 26.4% of patients had extraoral aphthae. Genital lesions have been described as infrequent,23 and lesions manifesting in other mucous membranes or on the skin are rare.24 A study reported genital involvement in 8% to 13% of patients with oral aphtha.25 We observed genital involvement in 25.6% of patients. Likewise, this higher value may be due to our study population of patients referred to our university hospital. In our study, 19.2% of patients presented with other inflammatory manifestations in addition to aphthous ulcerations (eg, folliculitis, EN, uveitis, arthritis). As dermatologists in a tertiary reference hospital, we actively look for such associations in every aphtha patient, which may not be the case in many nondermatologic oral care services.
In our study population, 43.2% of patients were diagnosed with or were under investigation for systemic diseases known to be associated with RAS. We found associations with Behçet disease most frequently, followed by IBD,26 solid organ transplantation, and HIV. In this group of patients, the respective systemic disease was active or poorly controlled. In transplant recipients, aphtha major was the most common type, similar to other studies.27 We observed no notable difference in the clinical picture of the oral ulcers in patients with a well-established systemic disease vs those without.
Most of our cases did not present findings other than aphtha, indicating that the intrinsic defect that predisposes to RAS is always systemic. Even mild and sporadic cases may be attributable to a systemic disorder of cutaneous-mucosal reactivity. The predisposition to RAS never originates in the oral cavity, hence the confusion caused and the uselessness of studies that relate aphthae to factors such as local food allergies, pH changes, or local infection with microorganisms.5,28 The disease course (reducing the frequency of lesion appearance and accelerating the healing of extensive lesions) is only modified with systemic treatment, with local measures proving to be only moderately useful to relieve pain. We believe that RAS can in many ways be compared to EN and pyoderma gangrenosum (PG): some systemic conditions that predispose patients to EN and PG also may predispose them to RAS (eg, IBD, hematologic disorders). Similar to RAS, many cases of EN and PG are idiopathic. In addition, pathergy also occurs in PG.11,13
We were unable to observe or establish any predictive clinical element that could indicate a better or worse response to the prescribed treatments, which also has been noted by other authors.3,4 Treatment of RAS is empiric, generally starting with drugs that are easier to prescribe and with fewer adverse effects, then progressing to more complex drugs when a good response is not obtained. Colchicine was the most commonly prescribed medication (76.4% [84/110]). It has been proposed by several authors3,4 as a first-line systemic medication for the treatment of recurrent aphthae, as it has been shown to be effective and safe. The dosage ranged from 0.5 mg twice daily to 0.5 mg 4 times daily. Dapsone is an established drug for aphtha29,30 and was used in 12 of our patients. The dosage used in our patients ranged from 50 to 100 mg/d. Adverse effects such as hemolytic anemia frequently are seen, and one of the patients in our study developed DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome in response to dapsone. In 7 cases, colchicine and dapsone were used together, which is believed to potentiate the therapeutic effects. This combination may be useful in patients for whom thalidomide cannot be used or those who have not improved with monotherapy.29 Thalidomide is considered one of the most effective drugs for RAS.30,31 Forty-three patients in our analysis were treated with thalidomide,usually as a first choice. The dosage ranged from 100 to 200 mg/d. It was mainly chosen in disabling pediatric cases, adult men with aphthous major, and women with no risk for pregnancy. Due to its potential adverse effects, thalidomide has been recommended when there is no response with other medications that are dose dependent; severe adverse effects such as thromboembolism and peripheral neuropathy are rare.31 Oral corticosteroids were used in 26 patients, aiming at rapid improvement in very symptomatic cases; however, due to the potential for long-term adverse effects, in all cases they were prescribed in combination with another medication that was maintained after the corticosteroid was discontinued.
We highlight the systemic nature of RAS as well as its frequent association with systemic diseases and other correlated manifestations (pustules, EN, arthralgia). We also emphasize the importance of using oral medications to adequately control the disease and do not recommend topical medications aimed at treating local causes. Dermatologists should be consulted in managing severe cases of RAS.
- Buño IJ, Huff JC, Weston WL, et al. Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch Dermatol. 1998;134:827-831.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728- 732.
- Natah SS, Konttinen YT, Enattah NS, et al. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221-234.
- Zunt SL. Recurrent aphthous stomatitis. Dermatol Clin. 2003;21:33-39.
- Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. number VI. recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21.
- Chams-Davatchi C, Shizarpour M, Davatchi F, et al. Comparison of oral aphthae in Behçet’s disease and idiopathic recurrent aphthous stomatitis. Adv Exp Med Biol. 2003;528:317-320.
- Schemel-Suárez M, López-López J, Chimenos-Küstner E. Oral ulcers: differential diagnosis and treatment [in Spanish]. Med Clin (Barc). 2015;145:499-503.
- S´lebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz). 2014;62:205-215.
- Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10:26-36.
- Jorizzo JL, Taylor RS, Schmalstieg FC, et al. Complex aphthosis: a forme fruste of Behçet’s syndrome? J Am Acad Dermatol. 1985;13:80-84.
- McCarty MA, Garton RA, Jorizzo JL. Complex aphthosis and Behçet’s disease. Dermatol Clin. 2003;21:41-48.
- Bulur I, Melrem O. Behçet disease: new aspects. Clin Dermatol. 2017;35:421-434.
- Cui RZ, Rogers RS 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34:475-481.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728-732.
- Ship II. Epidemiologic aspects of recurrent aphthous ulcerations. Oral Surg Oral Med Oral Pathol. 1972;33:400-406.
- Ship JA. Recurrent aphthous stomatitis. an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:141-147.
- Wilhelmsen NS, Weber R, Monteiro F, et al. Correlation between histocompatibility antigens and recurrent aphthous stomatitis in the Brazilian population. Braz J Otorhinolaryngol. 2009;75:426-431.
- S´lebioda Z, Dorocka-Bobkowska B. Systemic and environmental risk factors for recurrent aphthous stomatitis in a Polish cohort of patients. Postepy Dermatol Alergol. 2019;36:196-201.
- Ship JA, Chavez EM, Doerr PA, et al. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95-112.
- Brocklehurst P, Tickle M, Glenny AM, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database Syst Rev. 2012;12:CD005411.
- Belenguer-Guallar I, Jiménez-Soriano Y, Ariadna Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis. a literature review. J Clin Exp Dent. 2014;6:E168-E174.
- Bagán JV, Sanchis JM, Milián MA, et al. Recurrent aphthous stomatitis. a study of the clinical characteristics of lesions in 93 cases. J Oral Pathol Med. 1991;20:395-397.
- Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19:195-204.
- Scully C, Porter S. Recurrent aphthous stomatitis: current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18:21-27
- Chapel TA. Origins of penile ulcerations. Arch Androl. 1979; 3: 351-357.
- Lourenço SV, Hussein TP, Bologna SB, et al. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204-207.
- Nico MM, Brito AE, Martins LE, et al. Oral ulcers in an immunosuppressed 5-year-old boy. Clin Exp Dermatol. 2008;33:367-368.
- Trakji B, Baroudi K, Kharma Y. The effect of dietary habits on the development of the recurrent aphthous stomatitis. Niger Med J. 2012;53:9-11.
- Lynde CB, Bruce AJ, Rogers RS 3rd. Successful treatment of complex aphthosis with colchicine and dapsone. Arch Dermatol. 2009;145:273-276.
- Letsinger JA, McCarty MA, Jorizzo JL. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J Am Acad Dermatol. 2005(3 pt 1);52:500-508.
- Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. Medicine (Baltimore). 2010;89:176-182.
- Buño IJ, Huff JC, Weston WL, et al. Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch Dermatol. 1998;134:827-831.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728- 732.
- Natah SS, Konttinen YT, Enattah NS, et al. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221-234.
- Zunt SL. Recurrent aphthous stomatitis. Dermatol Clin. 2003;21:33-39.
- Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. number VI. recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21.
- Chams-Davatchi C, Shizarpour M, Davatchi F, et al. Comparison of oral aphthae in Behçet’s disease and idiopathic recurrent aphthous stomatitis. Adv Exp Med Biol. 2003;528:317-320.
- Schemel-Suárez M, López-López J, Chimenos-Küstner E. Oral ulcers: differential diagnosis and treatment [in Spanish]. Med Clin (Barc). 2015;145:499-503.
- S´lebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz). 2014;62:205-215.
- Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10:26-36.
- Jorizzo JL, Taylor RS, Schmalstieg FC, et al. Complex aphthosis: a forme fruste of Behçet’s syndrome? J Am Acad Dermatol. 1985;13:80-84.
- McCarty MA, Garton RA, Jorizzo JL. Complex aphthosis and Behçet’s disease. Dermatol Clin. 2003;21:41-48.
- Bulur I, Melrem O. Behçet disease: new aspects. Clin Dermatol. 2017;35:421-434.
- Cui RZ, Rogers RS 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34:475-481.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728-732.
- Ship II. Epidemiologic aspects of recurrent aphthous ulcerations. Oral Surg Oral Med Oral Pathol. 1972;33:400-406.
- Ship JA. Recurrent aphthous stomatitis. an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:141-147.
- Wilhelmsen NS, Weber R, Monteiro F, et al. Correlation between histocompatibility antigens and recurrent aphthous stomatitis in the Brazilian population. Braz J Otorhinolaryngol. 2009;75:426-431.
- S´lebioda Z, Dorocka-Bobkowska B. Systemic and environmental risk factors for recurrent aphthous stomatitis in a Polish cohort of patients. Postepy Dermatol Alergol. 2019;36:196-201.
- Ship JA, Chavez EM, Doerr PA, et al. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95-112.
- Brocklehurst P, Tickle M, Glenny AM, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database Syst Rev. 2012;12:CD005411.
- Belenguer-Guallar I, Jiménez-Soriano Y, Ariadna Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis. a literature review. J Clin Exp Dent. 2014;6:E168-E174.
- Bagán JV, Sanchis JM, Milián MA, et al. Recurrent aphthous stomatitis. a study of the clinical characteristics of lesions in 93 cases. J Oral Pathol Med. 1991;20:395-397.
- Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19:195-204.
- Scully C, Porter S. Recurrent aphthous stomatitis: current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18:21-27
- Chapel TA. Origins of penile ulcerations. Arch Androl. 1979; 3: 351-357.
- Lourenço SV, Hussein TP, Bologna SB, et al. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204-207.
- Nico MM, Brito AE, Martins LE, et al. Oral ulcers in an immunosuppressed 5-year-old boy. Clin Exp Dermatol. 2008;33:367-368.
- Trakji B, Baroudi K, Kharma Y. The effect of dietary habits on the development of the recurrent aphthous stomatitis. Niger Med J. 2012;53:9-11.
- Lynde CB, Bruce AJ, Rogers RS 3rd. Successful treatment of complex aphthosis with colchicine and dapsone. Arch Dermatol. 2009;145:273-276.
- Letsinger JA, McCarty MA, Jorizzo JL. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J Am Acad Dermatol. 2005(3 pt 1);52:500-508.
- Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. Medicine (Baltimore). 2010;89:176-182.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>Pinto</fileName> <TBEID>0C02F48F.SIG</TBEID> <TBUniqueIdentifier>NJ_0C02F48F</TBUniqueIdentifier> <newsOrJournal>Journal</newsOrJournal> <publisherName>Frontline Medical Communications Inc.</publisherName> <storyname>Pinto</storyname> <articleType>1</articleType> <TBLocation>Copyfitting-CT</TBLocation> <QCDate/> <firstPublished>20240408T080710</firstPublished> <LastPublished>20240408T080710</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240408T080710</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Nathalia Targa Pinto, MD; Silvia Vanessa Lourenço, DDS; Marcello</byline> <bylineText>Nathalia Targa Pinto, MD; Silvia Vanessa Lourenço, DDS; Marcello Menta Simonsen Nico, MD </bylineText> <bylineFull>Nathalia Targa Pinto, MD; Silvia Vanessa Lourenço, DDS; Marcello</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange>171-173</pageRange> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:"> <name/> <rightsInfo> <copyrightHolder> <name/> </copyrightHolder> <copyrightNotice/> </rightsInfo> </provider> <abstract/> <metaDescription>To the Editor:Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,1,2 round ulcerations of variable si</metaDescription> <articlePDF/> <teaserImage/> <title>Recurrent Aphthous Stomatitis: Clinical Experience From a University Hospital in Brazil</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth>April</pubPubdateMonth> <pubPubdateDay/> <pubVolume>113</pubVolume> <pubNumber>4</pubNumber> <wireChannels/> <primaryCMSID/> <CMSIDs> <CMSID>2161</CMSID> </CMSIDs> <keywords> <keyword>recurrent aphthouse stomatitis</keyword> </keywords> <seeAlsos/> <publications_g> <publicationData> <publicationCode>CT</publicationCode> <pubIssueName>April 2024</pubIssueName> <pubArticleType>Original Articles | 2161</pubArticleType> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Cutis</journalTitle> <journalFullTitle>Cutis</journalFullTitle> <copyrightStatement>Copyright 2015 Frontline Medical Communications Inc., Parsippany, NJ, USA. All rights reserved.</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">12</term> </publications> <sections> <term canonical="true">104</term> </sections> <topics> <term canonical="true">27442</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Recurrent Aphthous Stomatitis: Clinical Experience From a University Hospital in Brazil</title> <deck/> </itemMeta> <itemContent> <p>To the Editor:<br/><br/>Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,<sup>1,2</sup> round ulcerations of variable sizes with a tendency for recurrence, most commonly located in nonkeratinized areas of the oral mucosa. Pathergy commonly is observed.<sup>3</sup> Although many authors consider the terms <i>RAS</i> and<i>aphtha</i> to be synonymous,<sup>4,5</sup> differentiating the clinical lesion (aphthous ulceration) from the disease (aphtha or RAS) can be useful, as several other diseases can at times manifest with similar ulcers (called <i>aphthoid lesions</i>), such as pemphigus vulgaris, mucous membrane pemphigoid, and erythema multiforme.<sup>6</sup></p> <p>It is estimated that approximately 20% of individuals worldwide have at least one episode of aphtha during their lifetime,<sup>7</sup> and it is considered the most common disease of the oral mucosa.<sup>8,9</sup> However, only patients presenting with severe acute outbreaks or frequent relapses typically seek medical treatment. Clinically, aphthous ulcers are classified as <i>aphtha minor</i> (small number of small lesions), <i>aphtha major</i> (large deep lesions that also can affect the minor salivary glands with intense necrosis, difficulty in healing, and mucosal scarring), and <i>aphtha herpetiformis</i> (innumerous tiny lesions that reappear in recurring outbreaks).<sup>1-3</sup> The term <i>complex aphthosis</i> was introduced in 1985<sup>10</sup> and is defined as recurrent oral and genital aphthous ulcerations or recurring multiple oral aphthous ulcers in the absence of systemic manifestations or Behçet disease<sup>11,12</sup>; however, complex aphthosis also has been reported as frequent episodes of ulcerations that may be associated with systemic diseases including Behçet disease.<sup>13,14<br/><br/></sup>Currently, RAS is considered an immunologically mediated alteration in cutaneous mucosal reactivity with a multifactorial systemic cause. Underlying conditions such as Behçet disease, inflammatory bowel disease (IBD), iatrogenic immunosuppression (eg, following solid organ transplantation), AIDS, and cyclic neutropenia may or may not be detected.<sup>11-13</sup> <br/><br/>Our retrospective study explored the systemic nature of RAS. We reviewed patient records to evaluate underlying systemic conditions associated with the diagnosis of RAS and the use of oral medications in managing the disease. Medical records from the Department of Dermatology of the University of São Paulo, Brazil, from 2003 to 2017 were reviewed to identify patients with a diagnosis of RAS. Clinical classification of RAS—minor, major, or herpetiform—as well as the presence of aphthous lesions in other locations and the presence of other associated inflammatory cutaneous manifestations also were noted. Associated systemic diseases and treatments for RAS were recorded. Patients for whom the diagnosis of RAS was changed during follow-up were excluded. Because this was a retrospective analysis of medical records and without any patient risk, informed consent was not needed. <br/><br/>Medical records for 125 patients were reviewed; 63 were male (50.4%), and 62 were female (49.6%). The age at onset of symptoms, which ranged from a few months after birth to 74 years, was reported in only 92 (73.6%) patient medical records. Of these, 30 (32.6%) reported onset before 20 years of age, 39 (42.4%) between 20 and 39 years, 17 (18.5%) between 40 and 59 years, and 6 (6.5%) at 60 years or older. Morphologically, 72 (57.6%) had minor, 42 (33.6%) had major, and 11 (8.8%) had herpetiform aphthous ulcers. None of the patients presented with sporadic lesions; the disease was long-standing and persistent in all cases (complex aphthosis). <br/><br/>Regarding the location of the ulcers, 92 (73.6%) patients had lesions on the oral mucosa only. Some patients had lesions in more than one site in addition to the oral mucosa: 32 (25.6%) had aphthae in the genital/groin region and 4 (3.2%) presented with perianal/anal aphthae. Nineteen patients (19.2%) presented other cutaneous manifestations in addition to aphthae: 11 (45.8%) had folliculitis/pseudofolliculitis, and 8 (33.3%) had erythema nodosum (EN). Eight patients (33.3%) presented with uveitis, and 6 (25%) presented with concomitant arthralgia/arthritis. Fifty-four patients (43.2%) had confirmed or suspected associated disease: Behçet disease (21 [38.9%]), IBD (10 [18.5%]), solid organ transplantation (7 [13.0%])(kidney, 4 [57.1%]; heart, 2 [28.6%]; liver, 1 [14.3%]), HIV infection (6 [11.1%]), lymphoma (1 [1.9%]), aplastic anemia (1 [1.9%]), or myelodysplastic syndrome (1 [1.9%]). Ten patients (18.5%) presented with other diseases under investigation (eg, unidentified rheumatologic disease, unexplained neutropenia, undiagnosed immunodeficiencies, autoinflammatory syndromes, possible cyclic neutropenia). <br/><br/>Biopsies of the oral mucosa were performed in 31 patients. Histopathologic findings will be discussed in a future publication (unpublished data).<br/><br/>Five patients (4.0%) were lost to follow-up and did not receive treatment; 10 (8.0%) received only topical treatment (analgesics and/or corticosteroids). All 9 (7.2%) patients undergoing intralesional corticosteroid injections also were on a systemic treatment. One hundred ten (88.0%) patients were treated systemically—with colchicine (84/110 [76.4%]), thalidomide (43/110 [39.1%]), small pulses of oral corticosteroids (26 [23.6%]), dapsone (12/110 [10.9%]), or pentoxifylline (3 [2.7%]). Furthermore, in patients with associated diseases, treatment of the underlying condition was conducted when available, and follow-up was carried out in conjunction with the appropriate specialists. For treatment of the associated disease, patients received other medications such as methotrexate, azathioprine, cyclophosphamide, intravenous corticosteroid pulse, and immunobiologics.<br/><br/>The prevalence of RAS between sexes in our study population was similar (50.4% male; 49.6% female). Results from prior studies have been mixed; some reported a higher prevalence in females,<sup>15-18</sup> while others found no predilection for sex among patients diagnosed with RAS.<sup>19,20</sup> In our analysis, 75% of patients experienced symptoms of RAS before 40 years of age; in prior studies, up to 56% of patients experienced symptoms between the ages of 20 and 40 years.<sup>21,22<br/><br/></sup>In our study, 26.4% of patients had extraoral aphthae. Genital lesions have been described as infrequent,<sup>23</sup> and lesions manifesting in other mucous membranes or on the skin are rare.<sup>24</sup> A study reported genital involvement in 8% to 13% of patients with oral aphtha.<sup>25</sup> We observed genital involvement in 25.6% of patients. Likewise, this higher value may be due to our study population of patients referred to our university hospital. In our study, 19.2% of patients presented with other inflammatory manifestations in addition to aphthous ulcerations (eg, folliculitis, EN, uveitis, arthritis). As dermatologists in a tertiary reference hospital, we actively look for such associations in every aphtha patient, which may not be the case in many nondermatologic oral care services. <br/><br/>In our study population, 43.2% of patients were diagnosed with or were under investigation for systemic diseases known to be associated with RAS. We found associations with Behçet disease most frequently, followed by IBD,<sup>26</sup> solid organ transplantation, and HIV. In this group of patients, the respective systemic disease was active or poorly controlled. In transplant recipients, aphtha major was the most common type, similar to other studies.<sup>27</sup> We observed no notable difference in the clinical picture of the oral ulcers in patients with a well-established systemic disease vs those without. <br/><br/>Most of our cases did not present findings other than aphtha, indicating that the intrinsic defect that predisposes to RAS is always systemic. Even mild and sporadic cases may be attributable to a systemic disorder of cutaneous-mucosal reactivity. The predisposition to RAS never originates in the oral cavity, hence the confusion caused and the uselessness of studies that relate aphthae to factors such as local food allergies, pH changes, or local infection with microorganisms.<sup>5,28</sup> The disease course (reducing the frequency of lesion appearance and accelerating the healing of extensive lesions) is only modified with systemic treatment, with local measures proving to be only moderately useful to relieve pain. We believe that RAS can in many ways be compared to EN and pyoderma gangrenosum (PG): some systemic conditions that predispose patients to EN and PG also may predispose them to RAS (eg, IBD, hematologic disorders). Similar to RAS, many cases of EN and PG are idiopathic. In addition, pathergy also occurs in PG.<sup>11,13</sup> <br/><br/>We were unable to observe or establish any predictive clinical element that could indicate a better or worse response to the prescribed treatments, which also has been noted by other authors.<sup>3,4</sup> Treatment of RAS is empiric, generally starting with drugs that are easier to prescribe and with fewer adverse effects, then progressing to more complex drugs when a good response is not obtained. Colchicine was the most commonly prescribed medication (76.4% [84/110]). It has been proposed by several authors<sup>3,4</sup> as a first-line systemic medication for the treatment of recurrent aphthae, as it has been shown to be effective and safe. The dosage ranged from 0.5 mg twice daily to 0.5 mg 4 times daily. Dapsone is an established drug for aphtha<sup>29,30</sup> and was used in 12 of our patients. The dosage used in our patients ranged from 50 to 100 mg/d. Adverse effects such as hemolytic anemia frequently are seen, and one of the patients in our study developed DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome in response to dapsone. In 7 cases, colchicine and dapsone were used together, which is believed to potentiate the therapeutic effects. This combination may be useful in patients for whom thalidomide cannot be used or those who have not improved with monotherapy.<sup>29</sup> Thalidomide is considered one of the most effective drugs for RAS.<sup>30,31</sup> Forty-three patients in our analysis were treated with thalidomide,<sup> </sup>usually as a first choice. The dosage ranged from 100 to 200 mg/d. It was mainly chosen in disabling pediatric cases, adult men with aphthous major, and women with no risk for pregnancy. Due to its potential adverse effects, thalidomide has been recommended when there is no response with other medications that are dose dependent; severe adverse effects such as thromboembolism and peripheral neuropathy are rare.<sup>31</sup> Oral corticosteroids were used in 26 patients, aiming at rapid improvement in very symptomatic cases; however, due to the potential for long-term adverse effects, in all cases they were prescribed in combination with another medication that was maintained after the corticosteroid was discontinued.<br/><br/>We highlight the systemic nature of RAS as well as its frequent association with systemic diseases and other correlated manifestations (pustules, EN, arthralgia). We also emphasize the importance of using oral medications to adequately control the disease and do not recommend topical medications aimed at treating local causes. Dermatologists should be consulted in managing severe cases of RAS.</p> <h2>References</h2> <p class="reference"> 1. Buño IJ, Huff JC, Weston WL, et al. Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. <i>Arch Dermatol.</i> 1998;134:827-831. <br/><br/> 2. Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. <i>Pediatr Infect Dis J.</i> 2007;26:728- 732. <br/><br/> 3. Natah SS, <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=Konttinen%20YT%5BAuthor%5D&cauthor=true&cauthor_uid=15287304">Konttinen YT</a></span>, <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=Enattah%20NS%5BAuthor%5D&cauthor=true&cauthor_uid=15287304">Enattah NS</a></span>, et al. Recurrent aphthous ulcers today: a review of the growing knowledge. <span class="Hyperlink"><i><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=.+Recurrent+aphthous+ulcers+today%3A+a+review+of+the+growing+knowledge">Int J Oral Maxillofac Surg.</a></i></span><i> </i>2004;33:221-234.<br/><br/> 4. Zunt SL. Recurrent aphthous stomatitis. <i>Dermatol Clin.</i> 2003;21:33-39. <br/><br/> 5. Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. number VI. recurrent aphthous stomatitis. <i>Oral Dis.</i> 2006;12:1-21.<br/><br/> 6. Chams-Davatchi C, Shizarpour M, Davatchi F, et al. Comparison of oral aphthae in Behçet’s disease and idiopathic recurrent aphthous stomatitis. <i>Adv Exp Med Biol.</i> 2003;528:317-320.</p> <p class="reference"> 7. Schemel-Suárez M, López-López J, Chimenos-Küstner E. Oral ulcers: differential diagnosis and treatment [in Spanish]. <i>Med Clin (Barc).</i> 2015;145:499-503.<br/><br/> 8. S´lebioda Z, Szponar E, Kowalska A. <span class="highlight">Etiopathogenesis</span> of <span class="highlight">recurrent</span> <span class="highlight">aphthous stomatitis</span> and the <span class="highlight">role</span> of <span class="highlight">immunologic</span> <span class="highlight">aspects</span>: literature review. <span class="Hyperlink"><i><a href="https://www.ncbi.nlm.nih.gov/pubmed/24217985">Arch Immunol Ther Exp (Warsz).</a></i></span> 2014;62:205-215.<br/><br/> 9. Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. <i>J Clin Aesthet Dermatol.</i> 2017;10:26-36.<br/><br/>10. Jorizzo JL, Taylor RS, Schmalstieg FC, et al. Complex aphthosis: a forme fruste of Behçet’s syndrome? <i>J Am Acad Dermatol.</i> 1985;13:80-84.<br/><br/>11. McCarty MA, Garton RA, Jorizzo JL. Complex aphthosis and Behçet’s disease. <i>Dermatol Clin.</i> 2003;21:41-48.<br/><br/>12. Bulur I, Melrem O. Behçet disease: new aspects. <i>Clin Dermatol.</i> 2017;35:421-434.<br/><br/>13. Cui RZ, Rogers RS 3rd. Recurrent aphthous stomatitis. <i>Clin Dermatol.</i> 2016;34:475-481.<br/><br/>14. Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. <i>Pediatr Infect Dis J.</i> 2007;26:728-732. <br/><br/>15. Ship II. Epidemiologic aspects of recurrent aphthous ulcerations. <i>Oral Surg Oral Med Oral Pathol. </i>1972;33:400-406. <br/><br/>16. Ship JA. Recurrent aphthous stomatitis. an update. <i>Oral Surg Oral Med Oral Pathol Oral Radiol Endod.</i> 1996;81:141-147.<br/><br/>17. Wilhelmsen NS, <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=Weber%20R%5BAuthor%5D&cauthor=true&cauthor_uid=19649495">Weber R</a></span>, Monteiro F, et al. Correlation between histocompatibility antigens and <span class="highlight">recurrent</span> <span class="highlight">aphthous stomatitis</span> in the Brazilian population. <i>Braz J Otorhinolaryngol.</i> 2009;75:426-431.<br/><br/>18. S´lebioda Z, <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=Dorocka-Bobkowska%20B%5BAuthor%5D&cauthor=true&cauthor_uid=31320854">Dorocka-Bobkowska B</a></span>. <span class="highlight">Systemic</span> and environmental risk factors for recurrent <span class="highlight">aphthous</span> stomatitis in a Polish cohort of patients. <i>Postepy Dermatol Alergol.</i> 2019;36:196-201.<br/><br/>19. Ship JA, Chavez EM, Doerr PA, et al. Recurrent aphthous stomatitis. <i>Quintessence Int.</i> 2000;31:95-112.<br/><br/>20. Brocklehurst P, Tickle M, Glenny AM, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). <i>Cochrane Database Syst Rev.</i> 2012;12:CD005411.<br/><br/>21. Belenguer-Guallar I, Jiménez-Soriano Y, Ariadna Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis. a literature review. <i>J Clin Exp Dent.</i> 2014;6:E168-E174.<br/><br/>22. Bagán JV, Sanchis JM, Milián MA, et al. Recurrent aphthous stomatitis. a study of the clinical characteristics of lesions in 93 cases. <i>J Oral Pathol Med.</i> 1991;20:395-397. <br/><br/>23. Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. <i>J Pediatr Adolesc Gynecol.</i> 2006;19:195-204. <br/><br/>24. Scully C, Porter S. Recurrent aphthous stomatitis: current concepts of etiology, pathogenesis and management. <i>J Oral Pathol Med. </i>1989;18:21-27<br/><br/>25. Chapel TA. Origins of penile ulcerations. <i>Arch Androl.</i> 1979; 3: 351-357.<br/><br/>26. Lourenço SV, Hussein TP, Bologna SB, et al. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. <i>J Eur Acad Dermatol Venereol.</i> 2010;24:204-207.<br/><br/>27. Nico MM, Brito AE, Martins LE, et al. Oral ulcers in an immunosuppressed 5-year-old boy. <i>Clin Exp Dermatol.</i> 2008;33:367-368.<br/><br/>28. Trakji B, Baroudi K, Kharma Y. The effect of dietary habits on the development of the recurrent aphthous stomatitis. <i>Niger Med J.</i> 2012;53:9-11. <br/><br/>29. Lynde CB, Bruce AJ, Rogers RS 3rd. Successful treatment of complex aphthosis with colchicine and dapsone. <i>Arch Dermatol.</i> 2009;145:273-276. <br/><br/>30. <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=Letsinger%20JA%5BAuthor%5D&cauthor=true&cauthor_uid=15761429">Letsinger JA</a></span>, <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=McCarty%20MA%5BAuthor%5D&cauthor=true&cauthor_uid=15761429">McCarty MA</a></span>, <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pubmed/?term=Jorizzo%20JL%5BAuthor%5D&cauthor=true&cauthor_uid=15761429">Jorizzo JL</a></span>. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. <span class="Hyperlink"><i><a href="https://www.ncbi.nlm.nih.gov/pubmed/15761429">J Am Acad Dermatol.</a></i></span> 2005(3 pt 1);52:500-508.<br/><br/>31. Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. <i>Medicine (Baltimore).</i> 2010;89:176-182.</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>bio</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="disclosure">From the Universidade de São Paulo, Brazil. Drs. Pinto and Nico are from the Department of Dermatology, Faculdade de Medicina, and Dr. Lourenço is from the Department of Pathology, Faculdade de Odontologia.</p> <p class="disclosure">The authors report no conflict of interest. <br/><br/>Correspondence: Marcello Menta Simonsen Nico, MD, Departamento de Dermatologia, Faculdade de Medicina da Universidade de São Paulo, Brasil, R. Itapeva 500-3A. CEP-01332-000, São Paulo, Brasil (mentanico@hotmail.com).<br/><br/><em>Cutis.</em> 2024 April;113(4):171-173. doi:10.12788/cutis.0992.</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>in</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="insidehead">Practice <strong>Points</strong></p> <ul class="insidebody"> <li>The process that leads to the formation of aphthous ulcerations is always systemic, not local, even in the absence of a diagnosable systemic disease.</li> <li>Relapsing cases of aphthae should be treated with systemic medication.</li> </ul> </itemContent> </newsItem> </itemSet></root>
Practice Points
- The process that leads to the formation of aphthous ulcerations is always systemic, not local, even in the absence of a diagnosable systemic disease.
- Relapsing cases of aphthae should be treated with systemic medication.
Advancements in Targeted Therapies for Vitiligo: Prioritizing Equity in Drug Development
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).

Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).

Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).

Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>Delva</fileName> <TBEID>0C02F4C9.SIG</TBEID> <TBUniqueIdentifier>NJ_0C02F4C9</TBUniqueIdentifier> <newsOrJournal>Journal</newsOrJournal> <publisherName>Frontline Medical Communications Inc.</publisherName> <storyname>Delva</storyname> <articleType>1</articleType> <TBLocation>Copyfitting-CT</TBLocation> <QCDate/> <firstPublished>20240408T075813</firstPublished> <LastPublished>20240408T075813</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240408T075813</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Camile Delva, BS; Todd F. Pearson, PhD; John E. Harris, MD, PhD</byline> <bylineText>Camile Delva, BS; Todd F. Pearson, PhD; John E. Harris, MD, PhD</bylineText> <bylineFull>Camile Delva, BS; Todd F. Pearson, PhD; John E. Harris, MD, PhD</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange>156-158</pageRange> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:"> <name/> <rightsInfo> <copyrightHolder> <name/> </copyrightHolder> <copyrightNotice/> </rightsInfo> </provider> <abstract/> <metaDescription>Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 y</metaDescription> <articlePDF>300904</articlePDF> <teaserImage/> <title>Advancements in Targeted Therapies for Vitiligo: Prioritizing Equity in Drug Development</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth>April</pubPubdateMonth> <pubPubdateDay/> <pubVolume>113</pubVolume> <pubNumber>4</pubNumber> <wireChannels/> <primaryCMSID/> <CMSIDs> <CMSID>2159</CMSID> </CMSIDs> <keywords> <keyword>vitiligo</keyword> <keyword> pigmentation disorder</keyword> </keywords> <seeAlsos/> <publications_g> <publicationData> <publicationCode>CT</publicationCode> <pubIssueName>April 2024</pubIssueName> <pubArticleType>Departments | 2159</pubArticleType> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Cutis</journalTitle> <journalFullTitle>Cutis</journalFullTitle> <copyrightStatement>Copyright 2015 Frontline Medical Communications Inc., Parsippany, NJ, USA. All rights reserved.</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">12</term> </publications> <sections> <term canonical="true">136</term> </sections> <topics> <term canonical="true">276</term> </topics> <links> <link> <itemClass qcode="ninat:composite"/> <altRep contenttype="application/pdf">images/180026f6.pdf</altRep> <description role="drol:caption"/> <description role="drol:credit"/> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Advancements in Targeted Therapies for Vitiligo: Prioritizing Equity in Drug Development</title> <deck/> </itemMeta> <itemContent> <p class="abstract">Vitiligo is an autoimmune disease that causes depigmentation of the skin. It affects all skin types but can be especially disfiguring in patients with skin of color due to increased contrast of the depigmented patches compared to unaffected skin. The US Food and Drug Administration’s approval of ruxolitinib, a topical Janus kinase (JAK) inhibitor, has finally provided a treatment for vitiligo patients, but the lack of diversity among the study populations for JAK inhibitors must be addressed in future clinical trials.</p> <p>Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,<sup>1</sup> and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).<sup>2</sup> Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.<sup>3</sup> Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.<sup>4</sup> </p> <p>Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.<sup>5</sup> Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.<sup>6</sup> Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,<sup>7</sup> such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.<sup>5<br/><br/></sup>We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.</p> <h3>Autoimmune Pathogenesis and Treatment With JAK Inhibitors</h3> <p>Vitiligo is driven by autoreactive CD8<span class="body"><sup>+</sup></span> T cells that target melanocytes and secrete IFN-<span class="body">g</span>. Signaling of IFN-<span class="body">g</span> occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8<span class="body"><sup>+</sup></span> T cells at the epidermis where melanocytes reside.<sup>8</sup> Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.<sup>9,10</sup> </p> <p>Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.<sup>11</sup> <br/><br/>The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N<span class="body">=</span>330) and 30.9% of participants in TRuE-V2 (N<span class="body">=</span>344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).<sup>12</sup> In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.<sup>11</sup> Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.<sup>11</sup> Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).</p> <h3>Racial Disparities in Clinical Trials</h3> <p>Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States<sup>4</sup> even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib<sup>12</sup> and 2.7% of the participants in the phase 2b study of oral ritlecitinib.<sup>11</sup> This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.<sup>13</sup> </p> <h3>Efficacy Metrics Beyond Repigmentation</h3> <p>Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al<sup>14</sup> found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.<sup>15</sup> For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.<sup>1,11,12</sup> This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.<sup>1</sup></p> <h3>Considerations for Future Clinical Trials </h3> <p>It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications<sup>16,17</sup> outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.</p> <h2>References</h2> <p class="reference"> 1. Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. <i>J Eur Acad Dermatol Venereol. </i>2022;36:1831-1844.<br/><br/> 2. Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. <i>Nat Commun.</i> 2019;10:391.<br/><br/> 3. Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. <i>J Am Acad Dermatol.</i> 2017;77:1-13.<br/><br/> 4. Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. <i>JAMA Dermatol.</i> 2022;158:43-50.<br/><br/> 5. Spritz RA, Santorico SA. The genetic basis of vitiligo. <i>J Invest Dermatol. </i>2021;141:265-73.<br/><br/> 6. Harris JE. Chemical-induced vitiligo. <i>Dermatol Clin.</i> 2017;35:151-161.<br/><br/> 7. Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. <i>J Am Acad Dermatol.</i> 2023;88:945-948.<br/><br/> 8. Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. <i>Annu Rev Immunol</i>. 2020;38:621-648.<br/><br/> 9. Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. <i>Sci Transl Med.</i> 2018;10:<span class="cit">eaam7710</span>.<br/><br/>10. Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. <i>J Invest Dermatol.</i> 2019;139:769-778.<br/><br/>11. Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. <i>J Am Acad Dermatol.</i> 2023;88:395-403.<br/><br/>12. Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. <i>N Engl J Med.</i> 2022;387:1445-1455.<br/><br/>13. Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download<br/><br/>14. Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. <i>Br J Dermatol.</i> 2015;173:607-609.<br/><br/>15. Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. <i>Dermatol Clin.</i> 2017;35:117-128.<br/><br/>16. Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. <i>Cancer.</i> 2022;128:216-221.<br/><br/>17. Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. <i>Am J Mens Health.</i> 2023;17:15579883221147767.</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>bio</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="disclosure">Camile Delva is from the CUNY School of Medicine, New York, New York. Drs. Pearson and Harris are from the Department of Dermatology, UMass Chan Medical School, Worcester. </p> <p class="disclosure">Camile Delva and Dr. Pearson report no conflict of interest. Dr. Harris is a consultant for AbbVie, Incyte, and Pfizer, as well as an investigator and stockholder for Incyte.<br/><br/>Correspondence: John E. Harris, MD, PhD, Department of Dermatology, UMass Chan Medical School, 364 Plantation St, LRB 1010, Worcester, MA 01605 (John.Harris@umassmed.edu).<br/><br/><em>Cutis</em>. 2024 April;113(4):156-158. doi:10.12788/cutis.0995</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>in</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p class="insidehead">Practice <strong>Points</strong></p> <ul class="insidebody"> <li>Vitiligo is an autoimmune disease of the skin that affects all skin types but can be particularly disfiguring in those with skin of color. </li> <li>Ruxolitinib, a topical Janus kinase (JAK) inhibitor, is the only US Food and Drug Administration–approved treatment to repigment the skin in vitiligo and has shown efficacy for individuals with all skin phototypes.</li> </ul> </itemContent> </newsItem> </itemSet></root>
Practice Points
- Vitiligo is an autoimmune disease of the skin that affects all skin types but can be particularly disfiguring in those with skin of color.
- Ruxolitinib, a topical Janus kinase (JAK) inhibitor, is the only US Food and Drug Administration–approved treatment to repigment the skin in vitiligo and has shown efficacy for individuals with all skin phototypes.
- Individuals with skin of color are underrepresented in patient cohorts for JAK inhibitor clinical trials for vitiligo, mirroring a phenomenon seen in the majority of clinical trials. Ensuring diverse participant enrollment and measuring quality-of-life metrics will strengthen future clinical trials for treatment of vitiligo and other skin diseases impacting patients with skin of color.






