User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
Could Bedside Training Help End the US Neurologist Shortage?
DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.

DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.

DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167866</fileName> <TBEID>0C04FD4E.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FD4E</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>AAN: Neurologist Shortage</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240426T160833</QCDate> <firstPublished>20240426T160848</firstPublished> <LastPublished>20240426T160848</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240426T160848</CMSDate> <articleSource>FROM AAN 2024</articleSource> <facebookInfo/> <meetingNumber>2962-24</meetingNumber> <byline>Pauline Anderson</byline> <bylineText>PAULINE ANDERSON</bylineText> <bylineFull>PAULINE ANDERSON</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>A medical education initiative for internal medicine residents and medical students that offers instruction on assessing common neurologic conditions boosted tr</metaDescription> <articlePDF/> <teaserImage/> <teaser>As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns.</teaser> <title>Could Bedside Training Help End the US Neurologist Shortage?</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName>January 2021</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">22</term> <term>15</term> <term>21</term> </publications> <sections> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term canonical="true">38029</term> <term>258</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Could Bedside Training Help End the US Neurologist Shortage?</title> <deck/> </itemMeta> <itemContent> <p><span class="dateline">DENVER</span> — <span class="tag metaDescription">A medical education initiative for internal medicine residents and medical students that offers instruction on assessing common neurologic conditions boosted trainees’ confidence in caring for neurology patients and could help address the US neurologist shortage</span>, a new report suggested.<br/><br/>Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.<br/><br/>Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.<br/><br/>As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.<br/><br/>The findings were presented at the 2024 annual meeting of the American Academy of Neurology.<br/><br/></p> <h2>Addressing ‘Neurophobia’</h2> <p>Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”</p> <p>A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.<br/><br/>BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.<br/><br/>The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.<br/><br/>Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.<br/><br/>Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.<br/><br/>Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).<br/><br/>All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.<br/><br/>When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.<br/><br/>All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.<br/><br/></p> <h2>Use It or Lose It</h2> <p>Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.</p> <p>But time will tell if the newfound confidence of those taking the program actually lasts.<br/><br/>“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”<br/><br/>Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread <span class="Hyperlink"><a href="https://www.mdedge.com/neurology/article/265514/business-medicine/us-neurologist-shortage-insurmountable">neurologist shortage</a></span> in the United States.<br/><br/>A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.<br/><br/>“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.<br/><br/>Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.<br/><br/>He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”<br/><br/>The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.<br/><br/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/could-bedside-training-help-end-us-neurologist-shortage-2024a100085e">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM AAN 2024
Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167860</fileName> <TBEID>0C04FD2C.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FD2C</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240426T151917</QCDate> <firstPublished>20240426T152032</firstPublished> <LastPublished>20240426T152032</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240426T152032</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Deepa Varma</byline> <bylineText>DEEPA VARMA</bylineText> <bylineFull>DEEPA VARMA</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>A recent survey-based study found that only 4% of cancer survivors reported adhering to all four American Cancer Society (ACS) nutrition and physical activity g</metaDescription> <articlePDF/> <teaserImage/> <teaser>Researchers estimate more than 9,000 survivors’ adherence to weight, physical activity, fruit and vegetable consumption, and alcohol intake guidelines.</teaser> <title>Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>GIHOLD</publicationCode> <pubIssueName>January 2014</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term canonical="true">31</term> <term>18</term> <term>6</term> <term>15</term> <term>21</term> <term>23</term> <term>22</term> <term>13</term> </publications> <sections> <term canonical="true">27970</term> <term>39313</term> <term>86</term> </sections> <topics> <term>270</term> <term canonical="true">280</term> <term>198</term> <term>61821</term> <term>59244</term> <term>67020</term> <term>214</term> <term>217</term> <term>221</term> <term>238</term> <term>240</term> <term>242</term> <term>244</term> <term>39570</term> <term>245</term> <term>31848</term> <term>292</term> <term>178</term> <term>179</term> <term>181</term> <term>59374</term> <term>196</term> <term>197</term> <term>37637</term> <term>233</term> <term>243</term> <term>250</term> <term>49434</term> <term>303</term> <term>263</term> <term>192</term> <term>256</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines</title> <deck/> </itemMeta> <itemContent> <h2>TOPLINE:</h2> <p> <span class="tag metaDescription">A recent survey-based study found that only 4% of cancer survivors reported adhering to all four American Cancer Society (ACS) nutrition and physical activity guidelines, which include maintaining a healthy weight and diet, avoiding alcohol, and exercising regularly.</span> </p> <h2>METHODOLOGY:</h2> <ul class="body"> <li>The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.</li> <li>Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.</li> <li>The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.</li> <li>Overall, 9,121 survivors (91%) completed questionnaires for all four domains.</li> </ul> <h2>TAKEAWAY:</h2> <p>Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.<br/><br/>When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.<br/><br/>Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.<br/><br/>The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).</p> <h2>IN PRACTICE:</h2> <p>This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”</p> <h2>SOURCE:</h2> <p>This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamaoncology/fullarticle/2817661">online</a></span> in <em>JAMA Oncology</em>.</p> <h2>LIMITATIONS:</h2> <p>The authors reported several study limitations, most notably that self-reported data may introduce biases.</p> <h2>DISCLOSURES:</h2> <p>The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/few-cancer-survivors-meet-acs-nutrition-exercise-guidelines-2024a10007sl?src=">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Oregon Physician Assistants Get Name Change
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167861</fileName> <TBEID>0C04FD43.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FD43</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240426T111340</QCDate> <firstPublished>20240426T114737</firstPublished> <LastPublished>20240426T114737</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240426T114737</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Jennifer Nelson</byline> <bylineText>JENNIFER NELSON</bylineText> <bylineFull>JENNIFER NELSON</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>The switch is the first of its kind in the United States and comes on the heels of a decision from 2021 by the American Academy of Physician Associates (AAPA) t</metaDescription> <articlePDF/> <teaserImage/> <teaser>In June, Oregon PAs will be referred to as Physician Associates, a title change from Physician Assistants being debated nationwide. </teaser> <title>Oregon Physician Assistants Get Name Change</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>card</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>cpn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>idprac</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term>5</term> <term>6</term> <term>15</term> <term canonical="true">21</term> <term>9</term> <term>34</term> <term>13</term> <term>18</term> <term>20</term> <term>52226</term> <term>22</term> <term>23</term> <term>31</term> <term>25</term> <term>26</term> </publications> <sections> <term canonical="true">39313</term> </sections> <topics> <term canonical="true">38029</term> <term>278</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Oregon Physician Assistants Get Name Change</title> <deck/> </itemMeta> <itemContent> <p>On April 4, Oregon’s Governor Tina Kotek signed a <span class="Hyperlink"><a href="https://www.aapa.org/news-central/2024/04/oregon-governor-tina-kotek-signs-law-changing-pa-title/?utm_source=linkedin&utm_medium=aapa_post&utm_campaign=news_central">bill</a></span> into law that officially changed the title of “physician assistants” to “physician associates” in the state. <span class="tag metaDescription">The switch is the first of its kind in the United States and comes on the heels of a decision from 2021 by the American Academy of Physician Associates (AAPA) to change the meaning of “PA” to “physician associate” from “physician assistant.”</span></p> <p>In the <span class="Hyperlink"><a href="https://www.medscape.com/slideshow/2023-physician-assistant-satisfaction-6016503#2">Medscape Physician Assistant Career Satisfaction Report 2023</a>, </span>a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.<br/><br/>According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/985263">working independently</a></span> with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.<br/><br/>Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.<br/><br/>Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.<br/><br/>Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.<br/><br/>The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/oregon-physician-assistants-get-name-change-2024a100084h">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Vaccine Safety and DMT for Highly Active Multiple Sclerosis: New Data
, new research shows.
The study, the first to examine vaccine safety and immunogenicity in highly active MS, revealed high seroprotection rates following receipt of vaccines for COVID-19 and hepatitis A and B, regardless of the duration of treatment with natalizumab.
On the basis of these findings, investigators created an algorithm that clinicians can use to map an immunization schedule in patients who might otherwise delay initiation of disease-modifying therapy until they are fully vaccinated.
“We observed seroprotection rates exceeding 90% for hepatitis A and B, and mRNA COVID-19 vaccines, and all vaccines demonstrated a favorable safety profile, with no exacerbation of disease activity detected,” said lead author René Carvajal, MD, of the Department of Neurology-Neuroimmunology, Multiple Sclerosis Centre of Catalonia (Cemcat), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain. “This points to potential benefits for patients with highly active MS who require both immunization and high-efficacy therapies that may impact vaccine responses.”
The study was published online in JAMA Network Open.
A Controversial Issue
Today’s high-efficacy therapies for MS may increase the risk of acquiring new infections, reactivate latent pathogens, or worsen ongoing infectious conditions, and immunogenicity of vaccination can be compromised by immunosuppressive agents, particularly CD20 therapies, researchers noted.
As a result, many clinicians opt to delay initiation of such therapies until vaccination schedules are complete to avoid exposure to vaccine-preventable infections. But delaying treatment can potentially affect disease progression.
Reports of disease worsening following vaccination “have raised controversy around vaccine safety,” the authors wrote. The issue is especially relevant to those with highly active MS due to the scarcity of available data in this population.
The motivation for the study “stemmed from the complex balance clinicians face between initiating highly effective therapies promptly in patients with highly active MS and ensuring adequate protection against preventable infections through vaccination,” Dr. Carvajal said.
High Seroprotection Rate
Researchers analyzed data on 60 patients (mean age, 43 years; 44 female; mean disease duration, 17 years) participating in one of two prospectively followed cohorts: The Barcelona Clinically Isolated Syndromes Inception Cohort and the Barcelona Treatment Cohort. Data included demographic, clinical, radiologic, and biological data as well as regular clinical assessments, evaluations of the Expanded Disability Status Scale (EDSS), and MRI scans.
Patients enrolled in the current study had received at least one of these vaccines between September 2016 and February 2022: hepatitis A virus (HAV), hepatitis B virus (HBV; enhanced immunity high load or adjuvanted), or COVID-19 (BNT162b2 [Pfizer-BioNTech], mRAN-1273 [Moderna], or ChAdOx1-S [recombinant; AstraZeneca]).
The researchers conducted a retrospective, self-controlled analysis to compare the annualized relapse rate, EDSS score, and new T2 lesions counts during the 12 months before and after vaccination in patients with short- and long-term treatment duration.
They also compared John Cunningham virus serostatus between the two periods, as well as immunoglobulin G titers for each vaccine.
The global seroprotection rate was 93% (95% CI, 86%-98%). Individual vaccine rates were 92% for HAV, 93% for HBV, and 100% for COVID-19.
There was a significant reduction between the pre- and postvaccination periods in mean relapse rates (P = .004) and median number of new T2 lesions (P = .01).
There were no changes in EDSS scores before and after vaccinations and duration of natalizumab treatment had no impact on safety and immunogenicity.
‘Viable Option’
The researchers used their findings to create a proposed algorithm to inform immunization decisions in patients with highly active MS who require prompt initiation of high-efficacy disease-modifying therapy.
The algorithm is “integrated into a risk-minimization strategy tailored for patients with highly active MS, emphasizing in this case the pivotal role of natalizumab in averting treatment delays and providing adequate protection against potentially severe infections,” Dr. Carvajal said.
Participants who initiated or continued treatment with natalizumab completed their vaccination regimen without any incidents of progressive multifocal leukoencephalopathy (PML) or disease activity rebound following natalizumab discontinuation.
This suggests that using natalizumab for a brief duration might be a “viable option to contemplate,” the authors noted.
Commenting on the findings, Grace Gombolay, MD, assistant professor of pediatrics in the Division of Pediatric Neurology and director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic, Emory University, Atlanta, Georgia, said the study “demonstrates that vaccines are safe and do not trigger attacks in patients with MS on natalizumab, and that immunity — as measured by antibodies — is preserved in MS patients who receive natalizumab.”
This “contrasts with other treatments, as decreased antibody responses in COVID-19 are noted in certain treatments,” said Dr. Gombolay, who was not part of the study. “If both disease control and immunity against infection are the goals for the patient, then natalizumab is a reasonable option.”
“However, this must be balanced with other considerations,” she added, including the risk for PML and pregnancy.
This study was supported by grants from the European Committee for Treatment and Research in Multiple Sclerosis, Instituto de Salud Carlos III, and the European Union. Dr. Carvajal reported receiving grants from Vall d’Hebron Institut de Recerca and the European Committee for Treatment and Research in Multiple Sclerosis and honoraria from Roche, Novartis, BIIB-Colombia, Merck, and Sanofi outside the submitted work. Dr. Gombolay serves as media editor for Pediatric Neurology and as associate editor of the Annals of the Child Neurology Society. She is also a part-time CDC consultant for acute flaccid myelitis and received an honorarium as a speaker at the Georgia Neurological Society meeting, sponsored by Academic CME and TG Therapeutics.
A version of this article appeared on Medscape.com.
, new research shows.
The study, the first to examine vaccine safety and immunogenicity in highly active MS, revealed high seroprotection rates following receipt of vaccines for COVID-19 and hepatitis A and B, regardless of the duration of treatment with natalizumab.
On the basis of these findings, investigators created an algorithm that clinicians can use to map an immunization schedule in patients who might otherwise delay initiation of disease-modifying therapy until they are fully vaccinated.
“We observed seroprotection rates exceeding 90% for hepatitis A and B, and mRNA COVID-19 vaccines, and all vaccines demonstrated a favorable safety profile, with no exacerbation of disease activity detected,” said lead author René Carvajal, MD, of the Department of Neurology-Neuroimmunology, Multiple Sclerosis Centre of Catalonia (Cemcat), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain. “This points to potential benefits for patients with highly active MS who require both immunization and high-efficacy therapies that may impact vaccine responses.”
The study was published online in JAMA Network Open.
A Controversial Issue
Today’s high-efficacy therapies for MS may increase the risk of acquiring new infections, reactivate latent pathogens, or worsen ongoing infectious conditions, and immunogenicity of vaccination can be compromised by immunosuppressive agents, particularly CD20 therapies, researchers noted.
As a result, many clinicians opt to delay initiation of such therapies until vaccination schedules are complete to avoid exposure to vaccine-preventable infections. But delaying treatment can potentially affect disease progression.
Reports of disease worsening following vaccination “have raised controversy around vaccine safety,” the authors wrote. The issue is especially relevant to those with highly active MS due to the scarcity of available data in this population.
The motivation for the study “stemmed from the complex balance clinicians face between initiating highly effective therapies promptly in patients with highly active MS and ensuring adequate protection against preventable infections through vaccination,” Dr. Carvajal said.
High Seroprotection Rate
Researchers analyzed data on 60 patients (mean age, 43 years; 44 female; mean disease duration, 17 years) participating in one of two prospectively followed cohorts: The Barcelona Clinically Isolated Syndromes Inception Cohort and the Barcelona Treatment Cohort. Data included demographic, clinical, radiologic, and biological data as well as regular clinical assessments, evaluations of the Expanded Disability Status Scale (EDSS), and MRI scans.
Patients enrolled in the current study had received at least one of these vaccines between September 2016 and February 2022: hepatitis A virus (HAV), hepatitis B virus (HBV; enhanced immunity high load or adjuvanted), or COVID-19 (BNT162b2 [Pfizer-BioNTech], mRAN-1273 [Moderna], or ChAdOx1-S [recombinant; AstraZeneca]).
The researchers conducted a retrospective, self-controlled analysis to compare the annualized relapse rate, EDSS score, and new T2 lesions counts during the 12 months before and after vaccination in patients with short- and long-term treatment duration.
They also compared John Cunningham virus serostatus between the two periods, as well as immunoglobulin G titers for each vaccine.
The global seroprotection rate was 93% (95% CI, 86%-98%). Individual vaccine rates were 92% for HAV, 93% for HBV, and 100% for COVID-19.
There was a significant reduction between the pre- and postvaccination periods in mean relapse rates (P = .004) and median number of new T2 lesions (P = .01).
There were no changes in EDSS scores before and after vaccinations and duration of natalizumab treatment had no impact on safety and immunogenicity.
‘Viable Option’
The researchers used their findings to create a proposed algorithm to inform immunization decisions in patients with highly active MS who require prompt initiation of high-efficacy disease-modifying therapy.
The algorithm is “integrated into a risk-minimization strategy tailored for patients with highly active MS, emphasizing in this case the pivotal role of natalizumab in averting treatment delays and providing adequate protection against potentially severe infections,” Dr. Carvajal said.
Participants who initiated or continued treatment with natalizumab completed their vaccination regimen without any incidents of progressive multifocal leukoencephalopathy (PML) or disease activity rebound following natalizumab discontinuation.
This suggests that using natalizumab for a brief duration might be a “viable option to contemplate,” the authors noted.
Commenting on the findings, Grace Gombolay, MD, assistant professor of pediatrics in the Division of Pediatric Neurology and director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic, Emory University, Atlanta, Georgia, said the study “demonstrates that vaccines are safe and do not trigger attacks in patients with MS on natalizumab, and that immunity — as measured by antibodies — is preserved in MS patients who receive natalizumab.”
This “contrasts with other treatments, as decreased antibody responses in COVID-19 are noted in certain treatments,” said Dr. Gombolay, who was not part of the study. “If both disease control and immunity against infection are the goals for the patient, then natalizumab is a reasonable option.”
“However, this must be balanced with other considerations,” she added, including the risk for PML and pregnancy.
This study was supported by grants from the European Committee for Treatment and Research in Multiple Sclerosis, Instituto de Salud Carlos III, and the European Union. Dr. Carvajal reported receiving grants from Vall d’Hebron Institut de Recerca and the European Committee for Treatment and Research in Multiple Sclerosis and honoraria from Roche, Novartis, BIIB-Colombia, Merck, and Sanofi outside the submitted work. Dr. Gombolay serves as media editor for Pediatric Neurology and as associate editor of the Annals of the Child Neurology Society. She is also a part-time CDC consultant for acute flaccid myelitis and received an honorarium as a speaker at the Georgia Neurological Society meeting, sponsored by Academic CME and TG Therapeutics.
A version of this article appeared on Medscape.com.
, new research shows.
The study, the first to examine vaccine safety and immunogenicity in highly active MS, revealed high seroprotection rates following receipt of vaccines for COVID-19 and hepatitis A and B, regardless of the duration of treatment with natalizumab.
On the basis of these findings, investigators created an algorithm that clinicians can use to map an immunization schedule in patients who might otherwise delay initiation of disease-modifying therapy until they are fully vaccinated.
“We observed seroprotection rates exceeding 90% for hepatitis A and B, and mRNA COVID-19 vaccines, and all vaccines demonstrated a favorable safety profile, with no exacerbation of disease activity detected,” said lead author René Carvajal, MD, of the Department of Neurology-Neuroimmunology, Multiple Sclerosis Centre of Catalonia (Cemcat), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain. “This points to potential benefits for patients with highly active MS who require both immunization and high-efficacy therapies that may impact vaccine responses.”
The study was published online in JAMA Network Open.
A Controversial Issue
Today’s high-efficacy therapies for MS may increase the risk of acquiring new infections, reactivate latent pathogens, or worsen ongoing infectious conditions, and immunogenicity of vaccination can be compromised by immunosuppressive agents, particularly CD20 therapies, researchers noted.
As a result, many clinicians opt to delay initiation of such therapies until vaccination schedules are complete to avoid exposure to vaccine-preventable infections. But delaying treatment can potentially affect disease progression.
Reports of disease worsening following vaccination “have raised controversy around vaccine safety,” the authors wrote. The issue is especially relevant to those with highly active MS due to the scarcity of available data in this population.
The motivation for the study “stemmed from the complex balance clinicians face between initiating highly effective therapies promptly in patients with highly active MS and ensuring adequate protection against preventable infections through vaccination,” Dr. Carvajal said.
High Seroprotection Rate
Researchers analyzed data on 60 patients (mean age, 43 years; 44 female; mean disease duration, 17 years) participating in one of two prospectively followed cohorts: The Barcelona Clinically Isolated Syndromes Inception Cohort and the Barcelona Treatment Cohort. Data included demographic, clinical, radiologic, and biological data as well as regular clinical assessments, evaluations of the Expanded Disability Status Scale (EDSS), and MRI scans.
Patients enrolled in the current study had received at least one of these vaccines between September 2016 and February 2022: hepatitis A virus (HAV), hepatitis B virus (HBV; enhanced immunity high load or adjuvanted), or COVID-19 (BNT162b2 [Pfizer-BioNTech], mRAN-1273 [Moderna], or ChAdOx1-S [recombinant; AstraZeneca]).
The researchers conducted a retrospective, self-controlled analysis to compare the annualized relapse rate, EDSS score, and new T2 lesions counts during the 12 months before and after vaccination in patients with short- and long-term treatment duration.
They also compared John Cunningham virus serostatus between the two periods, as well as immunoglobulin G titers for each vaccine.
The global seroprotection rate was 93% (95% CI, 86%-98%). Individual vaccine rates were 92% for HAV, 93% for HBV, and 100% for COVID-19.
There was a significant reduction between the pre- and postvaccination periods in mean relapse rates (P = .004) and median number of new T2 lesions (P = .01).
There were no changes in EDSS scores before and after vaccinations and duration of natalizumab treatment had no impact on safety and immunogenicity.
‘Viable Option’
The researchers used their findings to create a proposed algorithm to inform immunization decisions in patients with highly active MS who require prompt initiation of high-efficacy disease-modifying therapy.
The algorithm is “integrated into a risk-minimization strategy tailored for patients with highly active MS, emphasizing in this case the pivotal role of natalizumab in averting treatment delays and providing adequate protection against potentially severe infections,” Dr. Carvajal said.
Participants who initiated or continued treatment with natalizumab completed their vaccination regimen without any incidents of progressive multifocal leukoencephalopathy (PML) or disease activity rebound following natalizumab discontinuation.
This suggests that using natalizumab for a brief duration might be a “viable option to contemplate,” the authors noted.
Commenting on the findings, Grace Gombolay, MD, assistant professor of pediatrics in the Division of Pediatric Neurology and director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic, Emory University, Atlanta, Georgia, said the study “demonstrates that vaccines are safe and do not trigger attacks in patients with MS on natalizumab, and that immunity — as measured by antibodies — is preserved in MS patients who receive natalizumab.”
This “contrasts with other treatments, as decreased antibody responses in COVID-19 are noted in certain treatments,” said Dr. Gombolay, who was not part of the study. “If both disease control and immunity against infection are the goals for the patient, then natalizumab is a reasonable option.”
“However, this must be balanced with other considerations,” she added, including the risk for PML and pregnancy.
This study was supported by grants from the European Committee for Treatment and Research in Multiple Sclerosis, Instituto de Salud Carlos III, and the European Union. Dr. Carvajal reported receiving grants from Vall d’Hebron Institut de Recerca and the European Committee for Treatment and Research in Multiple Sclerosis and honoraria from Roche, Novartis, BIIB-Colombia, Merck, and Sanofi outside the submitted work. Dr. Gombolay serves as media editor for Pediatric Neurology and as associate editor of the Annals of the Child Neurology Society. She is also a part-time CDC consultant for acute flaccid myelitis and received an honorarium as a speaker at the Georgia Neurological Society meeting, sponsored by Academic CME and TG Therapeutics.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167856</fileName> <TBEID>0C04FD1C.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FD1C</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>Vaccine DMT in MS</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240426T110304</QCDate> <firstPublished>20240426T112834</firstPublished> <LastPublished>20240426T112834</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240426T112833</CMSDate> <articleSource>FROM JAMA OPEN NETWORK</articleSource> <facebookInfo/> <meetingNumber/> <byline>Batya Swift Yasgur</byline> <bylineText>BATYA SWIFT YASGUR</bylineText> <bylineFull>BATYA SWIFT YASGUR</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Immunization with inactivated vaccines while receiving the natalizumab for highly active multiple sclerosis (MS) is safe and immunogenic, with no increased risk</metaDescription> <articlePDF/> <teaserImage/> <teaser>Investigators created an algorithm that clinicians can use to map an immunization schedule in patients who might otherwise delay initiation of disease-modifying therapy until they are fully vaccinated.</teaser> <title>Vaccine Safety and DMT for Highly Active Multiple Sclerosis: New Data</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName>January 2021</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>msrc</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term canonical="true">22</term> <term>59347</term> </publications> <sections> <term>39313</term> <term canonical="true">86</term> </sections> <topics> <term canonical="true">251</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Vaccine Safety and DMT for Highly Active Multiple Sclerosis: New Data</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">Immunization with inactivated vaccines while receiving the natalizumab for highly active multiple sclerosis (MS) is safe and immunogenic, with no increased risk for disease progression</span>, new research shows. </p> <p>The study, the first to examine vaccine safety and immunogenicity in highly active MS, revealed high seroprotection rates following receipt of vaccines for COVID-19 and hepatitis A and B, regardless of the duration of treatment with natalizumab.<br/><br/>On the basis of these findings, investigators created an algorithm that clinicians can use to map an immunization schedule in patients who might otherwise delay initiation of disease-modifying therapy until they are fully vaccinated.<br/><br/>“We observed seroprotection rates exceeding 90% for hepatitis A and B, and mRNA COVID-19 vaccines, and all vaccines demonstrated a favorable safety profile, with no exacerbation of disease activity detected,” said lead author René Carvajal, MD, of the Department of Neurology-Neuroimmunology, Multiple Sclerosis Centre of Catalonia (Cemcat), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain. “This points to potential benefits for patients with highly active MS who require both immunization and high-efficacy therapies that may impact vaccine responses.” <br/><br/>The study was <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2817477">published online</a></span> in <em>JAMA Network Open</em>.<br/><br/></p> <h2>A Controversial Issue</h2> <p>Today’s high-efficacy therapies for MS may increase the risk of acquiring new infections, reactivate latent pathogens, or worsen ongoing infectious conditions, and immunogenicity of vaccination can be compromised by immunosuppressive agents, particularly CD20 therapies, researchers noted.</p> <p>As a result, many clinicians opt to delay initiation of such therapies until vaccination schedules are complete to avoid exposure to vaccine-preventable infections. But delaying treatment can potentially affect disease progression. <br/><br/>Reports of disease worsening following vaccination “have raised controversy around vaccine safety,” the authors wrote. The issue is especially relevant to those with highly active MS due to the scarcity of available data in this population.<br/><br/>The motivation for the study “stemmed from the complex balance clinicians face between initiating highly effective therapies promptly in patients with highly active MS and ensuring adequate protection against preventable infections through vaccination,” Dr. Carvajal said.<br/><br/></p> <h2>High Seroprotection Rate</h2> <p>Researchers analyzed data on 60 patients (mean age, 43 years; 44 female; mean disease duration, 17 years) participating in one of two prospectively followed cohorts: The Barcelona Clinically Isolated Syndromes Inception Cohort and the Barcelona Treatment Cohort. Data included demographic, clinical, radiologic, and biological data as well as regular clinical assessments, evaluations of the Expanded Disability Status Scale (EDSS), and MRI scans.</p> <p>Patients enrolled in the current study had received at least one of these vaccines between September 2016 and February 2022: hepatitis A virus (HAV), hepatitis B virus (HBV; enhanced immunity high load or adjuvanted), or COVID-19 (BNT162b2 [Pfizer-BioNTech], mRAN-1273 [Moderna], or ChAdOx1-S [recombinant; AstraZeneca]).<br/><br/>The researchers conducted a retrospective, self-controlled analysis to compare the annualized relapse rate, EDSS score, and new T2 lesions counts during the 12 months before and after vaccination in patients with short- and long-term treatment duration.<br/><br/>They also compared John Cunningham virus serostatus between the two periods, as well as immunoglobulin G titers for each vaccine.<br/><br/>The global seroprotection rate was 93% (95% CI, 86%-98%). Individual vaccine rates were 92% for HAV, 93% for HBV, and 100% for COVID-19.<br/><br/>There was a significant reduction between the pre- and postvaccination periods in mean relapse rates (<em>P</em> = .004) and median number of new T2 lesions (<em>P</em> = .01).<br/><br/>There were no changes in EDSS scores before and after vaccinations and duration of natalizumab treatment had no impact on safety and immunogenicity.<br/><br/></p> <h2>‘Viable Option’</h2> <p>The researchers used their findings to create a proposed algorithm to inform immunization decisions in patients with highly active MS who require prompt initiation of high-efficacy disease-modifying therapy.</p> <p>The algorithm is “integrated into a risk-minimization strategy tailored for patients with highly active MS, emphasizing in this case the pivotal role of natalizumab in averting treatment delays and providing adequate protection against potentially severe infections,” Dr. Carvajal said.<br/><br/>Participants who initiated or continued treatment with natalizumab completed their vaccination regimen without any incidents of progressive multifocal leukoencephalopathy (PML) or disease activity rebound following natalizumab discontinuation.<br/><br/>This suggests that using natalizumab for a brief duration might be a “viable option to contemplate,” the authors noted.<br/><br/>Commenting on the findings, Grace Gombolay, MD, assistant professor of pediatrics in the Division of Pediatric Neurology and director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic, Emory University, Atlanta, Georgia, said the study “demonstrates that vaccines are safe and do not trigger attacks in patients with MS on natalizumab, and that immunity — as measured by antibodies — is preserved in MS patients who receive natalizumab.”<br/><br/>This “contrasts with other treatments, as decreased antibody responses in COVID-19 are noted in certain treatments,” said Dr. Gombolay, who was not part of the study. “If both disease control and immunity against infection are the goals for the patient, then natalizumab is a reasonable option.” <br/><br/>“However, this must be balanced with other considerations,” she added, including the risk for PML and pregnancy. <br/><br/>This study was supported by grants from the European Committee for Treatment and Research in Multiple Sclerosis, Instituto de Salud Carlos III, and the European Union. Dr. Carvajal reported receiving grants from Vall d’Hebron Institut de Recerca and the European Committee for Treatment and Research in Multiple Sclerosis and honoraria from Roche, Novartis, BIIB-Colombia, Merck, and Sanofi outside the submitted work. Dr. Gombolay serves as media editor for <em>Pediatric Neurology</em> and as associate editor of the <em>Annals of the Child Neurology Society</em>. She is also a part-time CDC consultant for acute flaccid myelitis and received an honorarium as a speaker at the Georgia Neurological Society meeting, sponsored by Academic CME and TG Therapeutics.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/vaccine-safety-and-dmt-highly-active-multiple-sclerosis-new-2024a10007zp">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM JAMA OPEN NETWORK
A Welcome Trade-off
At the end of March, in an anniversary no one but I noticed, I passed 4 years since I’d last rounded at the hospital.
It’s hard to comprehend that. I was at the hospital regularly for the first 22 years of my career, though admittedly it had dwindled from daily (1998-2011) to 1-2 weekends a month at the end.
Looking back, I still don’t miss it, and have no desire to go back. That’s not to say I don’t keep up on inpatient neurology, in case circumstances change, but at this point, honestly, I don’t want to. I’ve become accustomed to my non-hospital world, no late-night consults, no weekends spent rounding, no taking separate cars to restaurants or family events in case I get called in.
There are certainly things I miss about it. As odd as it may seem (and as much as I’d complain about it) I liked the wee hours of the really late night and early morning. It was quieter. Less chasing patients to tests or therapy. Pleasant idle chatter with staff and the few others docs around. Sitting at the computer and trying to think out a case on the fly. There was always junk food lying around.
But at this point in my life I’ll take the quiet of being home and my routine office hours. I know when my office day starts and ends. Aside from the occasional stop at Costco, I won’t be going anywhere else on my way home. I still get the occasional after-hours call, but none that require me to run to the ER.
On Fridays I’m glad the week is over, and don’t dread the 5:00 answering service switchover, or my call partner giving me the patient list.
There’s some revenue lost in the deal, but I’ll still take the trade-off.
It’s not like I ever had some grand plan to leave the hospital — I actually had thought I’d be there, at least occasionally, until retirement. But here I am.
Not to say there aren’t docs my age (and older) who still do it. Certainly our experience makes us good at it. But younger docs are closer to residency, which is primarily inpatient, so it’s an easier transition for many.
They probably have more energy, too.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
At the end of March, in an anniversary no one but I noticed, I passed 4 years since I’d last rounded at the hospital.
It’s hard to comprehend that. I was at the hospital regularly for the first 22 years of my career, though admittedly it had dwindled from daily (1998-2011) to 1-2 weekends a month at the end.
Looking back, I still don’t miss it, and have no desire to go back. That’s not to say I don’t keep up on inpatient neurology, in case circumstances change, but at this point, honestly, I don’t want to. I’ve become accustomed to my non-hospital world, no late-night consults, no weekends spent rounding, no taking separate cars to restaurants or family events in case I get called in.
There are certainly things I miss about it. As odd as it may seem (and as much as I’d complain about it) I liked the wee hours of the really late night and early morning. It was quieter. Less chasing patients to tests or therapy. Pleasant idle chatter with staff and the few others docs around. Sitting at the computer and trying to think out a case on the fly. There was always junk food lying around.
But at this point in my life I’ll take the quiet of being home and my routine office hours. I know when my office day starts and ends. Aside from the occasional stop at Costco, I won’t be going anywhere else on my way home. I still get the occasional after-hours call, but none that require me to run to the ER.
On Fridays I’m glad the week is over, and don’t dread the 5:00 answering service switchover, or my call partner giving me the patient list.
There’s some revenue lost in the deal, but I’ll still take the trade-off.
It’s not like I ever had some grand plan to leave the hospital — I actually had thought I’d be there, at least occasionally, until retirement. But here I am.
Not to say there aren’t docs my age (and older) who still do it. Certainly our experience makes us good at it. But younger docs are closer to residency, which is primarily inpatient, so it’s an easier transition for many.
They probably have more energy, too.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
At the end of March, in an anniversary no one but I noticed, I passed 4 years since I’d last rounded at the hospital.
It’s hard to comprehend that. I was at the hospital regularly for the first 22 years of my career, though admittedly it had dwindled from daily (1998-2011) to 1-2 weekends a month at the end.
Looking back, I still don’t miss it, and have no desire to go back. That’s not to say I don’t keep up on inpatient neurology, in case circumstances change, but at this point, honestly, I don’t want to. I’ve become accustomed to my non-hospital world, no late-night consults, no weekends spent rounding, no taking separate cars to restaurants or family events in case I get called in.
There are certainly things I miss about it. As odd as it may seem (and as much as I’d complain about it) I liked the wee hours of the really late night and early morning. It was quieter. Less chasing patients to tests or therapy. Pleasant idle chatter with staff and the few others docs around. Sitting at the computer and trying to think out a case on the fly. There was always junk food lying around.
But at this point in my life I’ll take the quiet of being home and my routine office hours. I know when my office day starts and ends. Aside from the occasional stop at Costco, I won’t be going anywhere else on my way home. I still get the occasional after-hours call, but none that require me to run to the ER.
On Fridays I’m glad the week is over, and don’t dread the 5:00 answering service switchover, or my call partner giving me the patient list.
There’s some revenue lost in the deal, but I’ll still take the trade-off.
It’s not like I ever had some grand plan to leave the hospital — I actually had thought I’d be there, at least occasionally, until retirement. But here I am.
Not to say there aren’t docs my age (and older) who still do it. Certainly our experience makes us good at it. But younger docs are closer to residency, which is primarily inpatient, so it’s an easier transition for many.
They probably have more energy, too.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167818</fileName> <TBEID>0C04FBE2.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FBE2</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>Hitting a Nerve: Trade-Off</storyname> <articleType>353</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240425T152540</QCDate> <firstPublished>20240425T152547</firstPublished> <LastPublished>20240425T152547</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240425T152547</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Allan M Block, MD</byline> <bylineText>ALLAN M. BLOCK, MD</bylineText> <bylineFull>ALLAN M. BLOCK, MD</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>Column</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Inpatient medicine these days, for better or worse, is a younger person’s game.</metaDescription> <articlePDF/> <teaserImage>170246</teaserImage> <teaser>Inpatient medicine these days, for better or worse, is a younger person’s game.</teaser> <title>A Welcome Trade-off</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName>January 2021</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">22</term> </publications> <sections> <term canonical="true">78</term> <term>39313</term> </sections> <topics> <term canonical="true">38029</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24005f83.jpg</altRep> <description role="drol:caption">Dr. Allan M. Block</description> <description role="drol:credit"/> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>A Welcome Trade-off</title> <deck/> </itemMeta> <itemContent> <p>At the end of March, in an anniversary no one but I noticed, I passed 4 years since I’d last rounded at the hospital.</p> <p>It’s hard to comprehend that. I was at the hospital regularly for the first 22 years of my career, though admittedly it had dwindled from daily (1998-2011) to 1-2 weekends a month at the end.<br/><br/>Looking back, I still don’t miss it, and have no desire to go back. That’s not to say I don’t keep up on inpatient neurology, in case circumstances change, but at this point, honestly, I don’t want to. I’ve become accustomed to my non-hospital world, no late-night consults, no weekends spent rounding, no taking separate cars to restaurants or family events in case I get called in.[[{"fid":"170246","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Allan M. Block, a neurologist in Scottsdale, Ariz.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"Dr. Allan M. Block"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_left"}}]]<br/><br/>There are certainly things I miss about it. As odd as it may seem (and as much as I’d complain about it) I liked the wee hours of the really late night and early morning. It was quieter. Less chasing patients to tests or therapy. Pleasant idle chatter with staff and the few others docs around. Sitting at the computer and trying to think out a case on the fly. There was always junk food lying around.<br/><br/>But at this point in my life I’ll take the quiet of being home and my routine office hours. I know when my office day starts and ends. Aside from the occasional stop at Costco, I won’t be going anywhere else on my way home. I still get the occasional after-hours call, but none that require me to run to the ER.<br/><br/>On Fridays I’m glad the week is over, and don’t dread the 5:00 answering service switchover, or my call partner giving me the patient list.<br/><br/>There’s some revenue lost in the deal, but I’ll still take the trade-off.<br/><br/>It’s not like I ever had some grand plan to leave the hospital — I actually had thought I’d be there, at least occasionally, until retirement. But here I am.<br/><br/><span class="tag metaDescription">Inpatient medicine these days, for better or worse, is a younger person’s game.</span> Not to say there aren’t docs my age (and older) who still do it. Certainly our experience makes us good at it. But younger docs are closer to residency, which is primarily inpatient, so it’s an easier transition for many.<br/><br/>They probably have more energy, too.<br/><br/></p> <p> <em>Dr. Block has a solo neurology practice in Scottsdale, Arizona.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167851</fileName> <TBEID>0C04FCF0.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FCF0</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>Published-All Pubs</TBLocation> <QCDate>20240425T104211</QCDate> <firstPublished>20240425T125615</firstPublished> <LastPublished>20240425T134723</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240425T125615</CMSDate> <articleSource>FROM JAMA DERMATOLOGY</articleSource> <facebookInfo/> <meetingNumber/> <byline>John Jesitus</byline> <bylineText>JOHN JESITUS</bylineText> <bylineFull>JOHN JESITUS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>a small, nonrandomized controlled trial, the injectable calcitonin gene-related peptide (CGRP) inhibitor erenumab significantly reduced treatment-resistant flus</metaDescription> <articlePDF/> <teaserImage>301181</teaserImage> <title>Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>2</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">13</term> <term>21</term> <term>15</term> <term>22</term> </publications> <sections> <term canonical="true">39313</term> <term>27970</term> </sections> <topics> <term canonical="true">291</term> <term>203</term> <term>258</term> <term>222</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012884.jpg</altRep> <description role="drol:caption">Persistent erythema in a patient with rosacea.</description> <description role="drol:credit">National Rosacea Society</description> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/240103e9.jpg</altRep> <description role="drol:caption">Dr. Emmy Graber</description> <description role="drol:credit"/> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012883.jpg</altRep> <description role="drol:caption">Dr. Guy F. Webster</description> <description role="drol:credit">Guy Webster, MD</description> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012789.jpg</altRep> <description role="drol:caption">Dr. John Barbieri</description> <description role="drol:credit">Brigham and Women's Hospital</description> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study</title> <deck/> </itemMeta> <itemContent> <p>In<span class="tag metaDescription"> a small, nonrandomized controlled trial, the injectable calcitonin gene-related peptide (CGRP) inhibitor erenumab significantly reduced treatment-resistant flushing and erythema associated with rosacea</span>. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.</p> <p>The <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamadermatology/article-abstract/2817738">study</a></span> was published in <em>JAMA Dermatology</em>.<br/><br/>[[{"fid":"301181","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Persistent erythema in a patient with rosacea.","field_file_image_credit[und][0][value]":"National Rosacea Society","field_file_image_caption[und][0][value]":"Persistent erythema in a patient with rosacea."},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” <span class="Hyperlink"><a href="https://www.dermboston.com/about-dermatologist-boston/emmy-graber/">Emmy Graber, MD, MBA</a></span>, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.[[{"fid":"281712","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Emmy Graber, a dermatologist in Boston","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"Dr. Emmy Graber"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_left"}}]]<br/><br/><span class="Hyperlink"><a href="https://www.websterdermatology.com/our-providers/guy-f-webster-md-phd-faad">Guy F. Webster, MD, PhD</a></span>, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.[[{"fid":"301182","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia","field_file_image_credit[und][0][value]":"Guy Webster, MD","field_file_image_caption[und][0][value]":"Dr. Guy F. Webster"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]<br/><br/></p> <h2>Spotlight on CGRP</h2> <p>Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamadermatology/fullarticle/2193990">study</a></span> published in <em>JAMA Dermatology</em> in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.<br/><br/>For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.<br/><br/>Participants received 3-monthly 140-mg doses of <span class="Hyperlink"><a href="https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761077s015lbl.pdf">erenumab</a></span>, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.<br/><br/>Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (<em>P</em> < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.<br/><br/></p> <h2>Questions Over QOL Data</h2> <p>“Although there were significant decreases in flushing and erythema,” wrote <span class="Hyperlink"><a href="https://physiciandirectory.brighamandwomens.org/details/15094/john-barbieri-dermatology-boston-chestnut_hill">John S. Barbieri, MD, MBA</a></span>, in an accompanying <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamadermatology/article-abstract/2817742">Editor’s Note</a></span>, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of <em>JAMA Dermatology</em>.<br/><br/>[[{"fid":"300850","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"John Barbieri, MD, MBA, assistant professor of dermatology, Harvard Medical School, and director of the Advanced Acne Therapeutics Clinic at Brigham and Women's Hospital, Boston","field_file_image_credit[und][0][value]":"Brigham and Women's Hospital","field_file_image_caption[und][0][value]":"Dr. John Barbieri"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_left"}}]]Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (<em>P</em> = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (<em>P</em> = .04 and .02).<br/><br/>No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.<br/><br/>However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.<br/><br/>Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”<br/><br/>Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.<br/><br/>Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.</p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/erenumab-reduces-persistent-flushing-and-erythema-rosacea-2024a10007vq?src=">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p>Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention.</p> </itemContent> </newsItem> </itemSet></root>
FROM JAMA DERMATOLOGY
Teleneurology for Suspected Stroke Speeds Treatment
, new research showed.
“This preliminary evidence supports adopting teleneurology prenotification as a best practice within health systems that have telestroke capabilities,” said study investigator Mark McDonald, MD, a neurologist at TeleSpecialists, Fort Myers, Florida.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Best Practices
The impact of emergency medical services prenotification, which refers to paramedics alerting receiving hospital emergency departments (EDs) of a suspected stroke on the way for appropriate preparations to be made, is well-defined, said Dr. McDonald.
“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”
Prenotification allows a neurologist to “get on the screen to begin coordinating with the ED team to adequately prepare for the possibility of thrombolytic treatment,” he added.
Currently, teleneurology prenotification, he said, is variable and its benefits unclear.
Dr. McDonald said “his organization, TeleSpecialists, maintains a large detailed medical records database for emergency-related, teleneurology, and other cases. For stroke, it recommends 15 best practices” for facilities including prenotification of teleneurology.
Other best practices include evaluating and administering thrombolysis in the CT imaging suite, a preassembled stroke kit that includes antihypertensives and thrombolytic agents, ensuring a weigh bed is available to determine the exact dose of thrombolysis treatment, and implementing “mock” stroke alerts, said Dr. McDonald.
From the database, researchers extracted acute telestroke consultations seen in the ED in 103 facilities in 15 states. Facilities that did not adhere to the 14 best practices other than teleneurologist prenotification were excluded from the analysis.
Of 9290 patients included in the study, 731 were treated with thrombolysis at prenotification facilities (median age, 69 years; median National Institutes of Health Stroke Score [NIHSS], 8) and 31 were treated at facilities without prenotification (median age, 63 years; median NIHSS score, 4). The thrombolytic treatment rate was 8.5% at prenotification facilities versus 4.8% at facilities without prenotification — a difference that was statistically significant.
Prenotification facilities had a significantly shorter median door-to-needle (DTN) time than those without such a process at 35 versus 43 minutes. In addition, there was a statistically significant difference in the percentage of patients with times less than 60 minutes at approximately 88% at prenotification facilities versus about 68% at the facilities without prenotification.
Case-Level Analysis
However, just because a facility adheres to teleneurology prenotification as a whole, doesn’t mean it occurs in every case. Researchers explored the impact of teleneurology prenotification at the case level rather than the facility level.
“That gave us a bit more insight into the real impact because it’s not just being at a facility with the best practice; it’s actually working case by case to see whether it happened or not and that’s where we get the most compelling findings,” said Dr. McDonald.
Of 761 treatment cases, there was prenotification to the neurology team in 401 cases. In 360 cases, prenotification did not occur.
The median DTN time was 29 minutes in the group with actual prenotification vs 41.5 minutes in the group without actual prenotification, a difference that was statistically significant, Dr. McDonald said.
As for treatment within 30 minutes of arrival, 50.4% of patients in the teleneurology prenotification group versus 18.9% in the no prenotification group — a statistically significant difference.
DTN time of less than 30 minutes is increasingly used as a target. “Being treated within this time frame improves outcomes and reduces length of hospital stay,” said Dr. McDonald.
The prenotification group also had a statistically significant higher percentage of treatment within 60 minutes of hospital arrival (93.5% vs 80%).
These new findings should help convince health and telestroke systems that teleneurology prenotification is worth implementing. “We want to achieve consensus on this as a best practice,” said Dr. McDonald.
Prenotification, he added, “coordinates the process and eliminates unnecessary and time-consuming steps.”
Dr. McDonald plans to prospectively study prenotification by collecting data on a facility before and after implementing a prenotification process.
Compelling Evidence
Commenting on the research, David L. Tirschwell, MD, Harborview Medical Center, Department of Neurology, Seattle, who cochaired the AAN session featuring the research, said the study provides compelling evidence that teleneurologist prenotification improves DTN time.
“Prenotifications are often standard of care in many healthcare settings and should likely be considered a best practice. When possible, extending such prenotification to a teleconsultant would make sense, and these preliminary data support that approach.”
However, more details are needed “to consider whether the intervention is possibly generalizable to other telestroke practices across the United States,” said Dr. Tirschwell.
Dr. McDonald reported receiving personal compensation for serving as a consultant for Syntrillo Inc. and has stock in Syntrillo Inc. Dr. Tirschwell reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, new research showed.
“This preliminary evidence supports adopting teleneurology prenotification as a best practice within health systems that have telestroke capabilities,” said study investigator Mark McDonald, MD, a neurologist at TeleSpecialists, Fort Myers, Florida.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Best Practices
The impact of emergency medical services prenotification, which refers to paramedics alerting receiving hospital emergency departments (EDs) of a suspected stroke on the way for appropriate preparations to be made, is well-defined, said Dr. McDonald.
“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”
Prenotification allows a neurologist to “get on the screen to begin coordinating with the ED team to adequately prepare for the possibility of thrombolytic treatment,” he added.
Currently, teleneurology prenotification, he said, is variable and its benefits unclear.
Dr. McDonald said “his organization, TeleSpecialists, maintains a large detailed medical records database for emergency-related, teleneurology, and other cases. For stroke, it recommends 15 best practices” for facilities including prenotification of teleneurology.
Other best practices include evaluating and administering thrombolysis in the CT imaging suite, a preassembled stroke kit that includes antihypertensives and thrombolytic agents, ensuring a weigh bed is available to determine the exact dose of thrombolysis treatment, and implementing “mock” stroke alerts, said Dr. McDonald.
From the database, researchers extracted acute telestroke consultations seen in the ED in 103 facilities in 15 states. Facilities that did not adhere to the 14 best practices other than teleneurologist prenotification were excluded from the analysis.
Of 9290 patients included in the study, 731 were treated with thrombolysis at prenotification facilities (median age, 69 years; median National Institutes of Health Stroke Score [NIHSS], 8) and 31 were treated at facilities without prenotification (median age, 63 years; median NIHSS score, 4). The thrombolytic treatment rate was 8.5% at prenotification facilities versus 4.8% at facilities without prenotification — a difference that was statistically significant.
Prenotification facilities had a significantly shorter median door-to-needle (DTN) time than those without such a process at 35 versus 43 minutes. In addition, there was a statistically significant difference in the percentage of patients with times less than 60 minutes at approximately 88% at prenotification facilities versus about 68% at the facilities without prenotification.
Case-Level Analysis
However, just because a facility adheres to teleneurology prenotification as a whole, doesn’t mean it occurs in every case. Researchers explored the impact of teleneurology prenotification at the case level rather than the facility level.
“That gave us a bit more insight into the real impact because it’s not just being at a facility with the best practice; it’s actually working case by case to see whether it happened or not and that’s where we get the most compelling findings,” said Dr. McDonald.
Of 761 treatment cases, there was prenotification to the neurology team in 401 cases. In 360 cases, prenotification did not occur.
The median DTN time was 29 minutes in the group with actual prenotification vs 41.5 minutes in the group without actual prenotification, a difference that was statistically significant, Dr. McDonald said.
As for treatment within 30 minutes of arrival, 50.4% of patients in the teleneurology prenotification group versus 18.9% in the no prenotification group — a statistically significant difference.
DTN time of less than 30 minutes is increasingly used as a target. “Being treated within this time frame improves outcomes and reduces length of hospital stay,” said Dr. McDonald.
The prenotification group also had a statistically significant higher percentage of treatment within 60 minutes of hospital arrival (93.5% vs 80%).
These new findings should help convince health and telestroke systems that teleneurology prenotification is worth implementing. “We want to achieve consensus on this as a best practice,” said Dr. McDonald.
Prenotification, he added, “coordinates the process and eliminates unnecessary and time-consuming steps.”
Dr. McDonald plans to prospectively study prenotification by collecting data on a facility before and after implementing a prenotification process.
Compelling Evidence
Commenting on the research, David L. Tirschwell, MD, Harborview Medical Center, Department of Neurology, Seattle, who cochaired the AAN session featuring the research, said the study provides compelling evidence that teleneurologist prenotification improves DTN time.
“Prenotifications are often standard of care in many healthcare settings and should likely be considered a best practice. When possible, extending such prenotification to a teleconsultant would make sense, and these preliminary data support that approach.”
However, more details are needed “to consider whether the intervention is possibly generalizable to other telestroke practices across the United States,” said Dr. Tirschwell.
Dr. McDonald reported receiving personal compensation for serving as a consultant for Syntrillo Inc. and has stock in Syntrillo Inc. Dr. Tirschwell reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, new research showed.
“This preliminary evidence supports adopting teleneurology prenotification as a best practice within health systems that have telestroke capabilities,” said study investigator Mark McDonald, MD, a neurologist at TeleSpecialists, Fort Myers, Florida.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Best Practices
The impact of emergency medical services prenotification, which refers to paramedics alerting receiving hospital emergency departments (EDs) of a suspected stroke on the way for appropriate preparations to be made, is well-defined, said Dr. McDonald.
“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”
Prenotification allows a neurologist to “get on the screen to begin coordinating with the ED team to adequately prepare for the possibility of thrombolytic treatment,” he added.
Currently, teleneurology prenotification, he said, is variable and its benefits unclear.
Dr. McDonald said “his organization, TeleSpecialists, maintains a large detailed medical records database for emergency-related, teleneurology, and other cases. For stroke, it recommends 15 best practices” for facilities including prenotification of teleneurology.
Other best practices include evaluating and administering thrombolysis in the CT imaging suite, a preassembled stroke kit that includes antihypertensives and thrombolytic agents, ensuring a weigh bed is available to determine the exact dose of thrombolysis treatment, and implementing “mock” stroke alerts, said Dr. McDonald.
From the database, researchers extracted acute telestroke consultations seen in the ED in 103 facilities in 15 states. Facilities that did not adhere to the 14 best practices other than teleneurologist prenotification were excluded from the analysis.
Of 9290 patients included in the study, 731 were treated with thrombolysis at prenotification facilities (median age, 69 years; median National Institutes of Health Stroke Score [NIHSS], 8) and 31 were treated at facilities without prenotification (median age, 63 years; median NIHSS score, 4). The thrombolytic treatment rate was 8.5% at prenotification facilities versus 4.8% at facilities without prenotification — a difference that was statistically significant.
Prenotification facilities had a significantly shorter median door-to-needle (DTN) time than those without such a process at 35 versus 43 minutes. In addition, there was a statistically significant difference in the percentage of patients with times less than 60 minutes at approximately 88% at prenotification facilities versus about 68% at the facilities without prenotification.
Case-Level Analysis
However, just because a facility adheres to teleneurology prenotification as a whole, doesn’t mean it occurs in every case. Researchers explored the impact of teleneurology prenotification at the case level rather than the facility level.
“That gave us a bit more insight into the real impact because it’s not just being at a facility with the best practice; it’s actually working case by case to see whether it happened or not and that’s where we get the most compelling findings,” said Dr. McDonald.
Of 761 treatment cases, there was prenotification to the neurology team in 401 cases. In 360 cases, prenotification did not occur.
The median DTN time was 29 minutes in the group with actual prenotification vs 41.5 minutes in the group without actual prenotification, a difference that was statistically significant, Dr. McDonald said.
As for treatment within 30 minutes of arrival, 50.4% of patients in the teleneurology prenotification group versus 18.9% in the no prenotification group — a statistically significant difference.
DTN time of less than 30 minutes is increasingly used as a target. “Being treated within this time frame improves outcomes and reduces length of hospital stay,” said Dr. McDonald.
The prenotification group also had a statistically significant higher percentage of treatment within 60 minutes of hospital arrival (93.5% vs 80%).
These new findings should help convince health and telestroke systems that teleneurology prenotification is worth implementing. “We want to achieve consensus on this as a best practice,” said Dr. McDonald.
Prenotification, he added, “coordinates the process and eliminates unnecessary and time-consuming steps.”
Dr. McDonald plans to prospectively study prenotification by collecting data on a facility before and after implementing a prenotification process.
Compelling Evidence
Commenting on the research, David L. Tirschwell, MD, Harborview Medical Center, Department of Neurology, Seattle, who cochaired the AAN session featuring the research, said the study provides compelling evidence that teleneurologist prenotification improves DTN time.
“Prenotifications are often standard of care in many healthcare settings and should likely be considered a best practice. When possible, extending such prenotification to a teleconsultant would make sense, and these preliminary data support that approach.”
However, more details are needed “to consider whether the intervention is possibly generalizable to other telestroke practices across the United States,” said Dr. Tirschwell.
Dr. McDonald reported receiving personal compensation for serving as a consultant for Syntrillo Inc. and has stock in Syntrillo Inc. Dr. Tirschwell reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167846</fileName> <TBEID>0C04FCAA.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FCAA</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>AAN: Teleneurology Stroke</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T145552</QCDate> <firstPublished>20240424T151203</firstPublished> <LastPublished>20240424T151203</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T151203</CMSDate> <articleSource>FROM AAN 2024</articleSource> <facebookInfo/> <meetingNumber>2962-24</meetingNumber> <byline>Pauline Anderson</byline> <bylineText>PAULINE ANDERSON</bylineText> <bylineFull>PAULINE ANDERSON</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Alerting neurologists via telemedicine that a patient with suspected acute stroke is en route to the hospital significantly enhances the speed at which thrombol</metaDescription> <articlePDF/> <teaserImage/> <teaser>“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”</teaser> <title>Teleneurology for Suspected Stroke Speeds Treatment</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName>January 2021</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>CARD</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle>Cardiology news</journalFullTitle> <copyrightStatement/> </publicationData> <publicationData> <publicationCode>EM</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term canonical="true">22</term> <term>5</term> <term>14</term> </publications> <sections> <term>39313</term> <term canonical="true">53</term> </sections> <topics> <term canonical="true">301</term> <term>258</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Teleneurology for Suspected Stroke Speeds Treatment</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">Alerting neurologists via telemedicine that a patient with suspected acute stroke is en route to the hospital significantly enhances the speed at which thrombolysis is administered and increases the number of patients who receive timelier, potentially lifesaving treatment</span>, new research showed.</p> <p>“This preliminary evidence supports adopting teleneurology prenotification as a best practice within health systems that have telestroke capabilities,” said study investigator Mark McDonald, MD, a neurologist at TeleSpecialists, Fort Myers, Florida.<br/><br/>The findings were presented at the 2024 annual meeting of the American Academy of Neurology.<br/><br/></p> <h2>Best Practices</h2> <p>The impact of emergency medical services prenotification, which refers to paramedics alerting receiving hospital emergency departments (EDs) of a suspected stroke on the way for appropriate preparations to be made, is well-defined, said Dr. McDonald.</p> <p>“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”<br/><br/>Prenotification allows a neurologist to “get on the screen to begin coordinating with the ED team to adequately prepare for the possibility of thrombolytic treatment,” he added.<br/><br/>Currently, teleneurology prenotification, he said, is variable and its benefits unclear.<br/><br/>Dr. McDonald said “his organization, TeleSpecialists, maintains a large detailed medical records database for emergency-related, teleneurology, and other cases. For stroke, it recommends 15 best practices” for facilities including prenotification of teleneurology.<br/><br/>Other best practices include evaluating and administering thrombolysis in the CT imaging suite, a preassembled stroke kit that includes antihypertensives and thrombolytic agents, ensuring a weigh bed is available to determine the exact dose of thrombolysis treatment, and implementing “mock” stroke alerts, said Dr. McDonald.<br/><br/>From the database, researchers extracted acute telestroke consultations seen in the ED in 103 facilities in 15 states. Facilities that did not adhere to the 14 best practices other than teleneurologist prenotification were excluded from the analysis.<br/><br/>Of 9290 patients included in the study, 731 were treated with thrombolysis at prenotification facilities (median age, 69 years; median National Institutes of Health Stroke Score [NIHSS], 8) and 31 were treated at facilities without prenotification (median age, 63 years; median NIHSS score, 4). The thrombolytic treatment rate was 8.5% at prenotification facilities versus 4.8% at facilities without prenotification — a difference that was statistically significant.<br/><br/>Prenotification facilities had a significantly shorter median door-to-needle (DTN) time than those without such a process at 35 versus 43 minutes. In addition, there was a statistically significant difference in the percentage of patients with times less than 60 minutes at approximately 88% at prenotification facilities versus about 68% at the facilities without prenotification.<br/><br/></p> <h2>Case-Level Analysis</h2> <p>However, just because a facility adheres to teleneurology prenotification as a whole, doesn’t mean it occurs in every case. Researchers explored the impact of teleneurology prenotification at the case level rather than the facility level.</p> <p>“That gave us a bit more insight into the real impact because it’s not just being at a facility with the best practice; it’s actually working case by case to see whether it happened or not and that’s where we get the most compelling findings,” said Dr. McDonald.<br/><br/>Of 761 treatment cases, there was prenotification to the neurology team in 401 cases. In 360 cases, prenotification did not occur.<br/><br/>The median DTN time was 29 minutes in the group with actual prenotification vs 41.5 minutes in the group without actual prenotification, a difference that was statistically significant, Dr. McDonald said.<br/><br/>As for treatment within 30 minutes of arrival, 50.4% of patients in the teleneurology prenotification group versus 18.9% in the no prenotification group — a statistically significant difference.<br/><br/>DTN time of less than 30 minutes is increasingly used as a target. “Being treated within this time frame improves outcomes and reduces length of hospital stay,” said Dr. McDonald.<br/><br/>The prenotification group also had a statistically significant higher percentage of treatment within 60 minutes of hospital arrival (93.5% vs 80%).<br/><br/>These new findings should help convince health and telestroke systems that teleneurology prenotification is worth implementing. “We want to achieve consensus on this as a best practice,” said Dr. McDonald.<br/><br/>Prenotification, he added, “coordinates the process and eliminates unnecessary and time-consuming steps.”<br/><br/>Dr. McDonald plans to prospectively study prenotification by collecting data on a facility before and after implementing a prenotification process.<br/><br/></p> <h2>Compelling Evidence</h2> <p>Commenting on the research, David L. Tirschwell, MD, Harborview Medical Center, Department of Neurology, Seattle, who cochaired the AAN session featuring the research, said the study provides compelling evidence that teleneurologist prenotification improves DTN time.</p> <p>“Prenotifications are often standard of care in many healthcare settings and should likely be considered a best practice. When possible, extending such prenotification to a teleconsultant would make sense, and these preliminary data support that approach.”<br/><br/>However, more details are needed “to consider whether the intervention is possibly generalizable to other telestroke practices across the United States,” said Dr. Tirschwell.<br/><br/>Dr. McDonald reported receiving personal compensation for serving as a consultant for Syntrillo Inc. and has stock in Syntrillo Inc. Dr. Tirschwell reported no relevant conflicts of interest.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/teleneurology-suspected-stroke-speeds-treatment-2024a10007yz">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM AAN 2024
Novel Agent Curbs Alzheimer’s-Related Agitation
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167847</fileName> <TBEID>0C04FCB7.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FCB7</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>AAN: Alzheimer's Agitation</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T142611</QCDate> <firstPublished>20240424T144904</firstPublished> <LastPublished>20240424T144904</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T144904</CMSDate> <articleSource>FROM AAN 2024</articleSource> <facebookInfo/> <meetingNumber>2962-24</meetingNumber> <byline>Megan Brooks</byline> <bylineText>MEGAN BROOKS</bylineText> <bylineFull>MEGAN BROOKS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Treatment with AXS-05, a combination of dextromethorphan and bupropion, demonstrated rapid, sustained, and clinically meaningful improvement in agitation relate</metaDescription> <articlePDF/> <teaserImage/> <teaser>More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo. </teaser> <title>Novel Agent Curbs Alzheimer’s-Related Agitation</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2024</pubPubdateYear> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>CPN</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> <publicationData> <publicationCode>FP</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>Copyright 2017 Frontline Medical News</copyrightStatement> </publicationData> <publicationData> <publicationCode>IM</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName>January 2021</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> </publications_g> <publications> <term>9</term> <term>15</term> <term>21</term> <term canonical="true">22</term> </publications> <sections> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term canonical="true">180</term> <term>258</term> <term>215</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Novel Agent Curbs Alzheimer’s-Related Agitation</title> <deck/> </itemMeta> <itemContent> <p><span class="dateline">DENVER </span>— <span class="tag metaDescription">Treatment with AXS-05, a combination of d<span class="Hyperlink">extromethorphan </span>and bupropion<span class="Hyperlink">,</span> demonstrated rapid, sustained, and clinically meaningful improvement in agitation related to Alzheimer’s disease and was generally well tolerated in the phase 3 ACCORD trial.</span> </p> <p>More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo. <br/><br/>“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York. <br/><br/>The findings were presented at the 2024 annual meeting of the American Academy of Neurology. <br/><br/></p> <h2>Common and Disruptive</h2> <p>Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.</p> <p>A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo. <br/><br/>ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation. <br/><br/>In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.<br/><br/>A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.<br/><br/>In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; <em>P</em> = .014).<br/><br/>“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported. <br/><br/>AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; <em>P</em> = .018).<br/><br/>Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture<span class="Hyperlink">)</span>.<br/><br/>Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.<br/><br/>One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation. <br/><br/></p> <h2>Promising Agent </h2> <p>Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.</p> <p>“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.<br/><br/>He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions. <br/><br/>“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained. <br/><br/>The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist. <br/><br/>“These medications can help reduce the risk of agitation,” Dr. Finney said. <br/><br/>“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added. <br/><br/>Last May, the US Food and Drug Administration (FDA) approved the antipsychotic b<span class="Hyperlink">rexpiprazole </span>(Rexulti) for Alzheimer’s disease-related agitation, making it the <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/991851">first FDA-approved drug</a></span> for this indication. <br/><br/>The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.<br/><br/>“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association. <br/><br/>Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”<br/><br/>The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/novel-agent-curbs-alzheimers-related-agitation-2024a10007ug">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM AAN 2024
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167842</fileName> <TBEID>0C04FC9A.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FC9A</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T122750</QCDate> <firstPublished>20240424T122826</firstPublished> <LastPublished>20240424T122826</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T122826</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Steph Weber</byline> <bylineText>STEPH WEBER</bylineText> <bylineFull>STEPH WEBER</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>Feature</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>The Federal Trade Commission (FTC) voted Tuesday to ban noncompete agreements, possibly making it easier for doctors to switch employers without having to leave</metaDescription> <articlePDF/> <teaserImage/> <teaser>But dissenting commissioners dispute the FTC’s authority to broadly ban noncompetes.</teaser> <title>Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>card</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>cpn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>idprac</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>icymicov</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdemed</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> <publicationData> <publicationCode>mdid</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>GIHOLD</publicationCode> <pubIssueName>January 2014</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term>5</term> <term>34</term> <term>6</term> <term>9</term> <term>13</term> <term canonical="true">15</term> <term>18</term> <term>20</term> <term>21</term> <term>26</term> <term>31</term> <term>23</term> <term>25</term> <term>22</term> <term>52226</term> <term>69586</term> <term>58877</term> <term>51892</term> </publications> <sections> <term canonical="true">27980</term> <term>39313</term> <term>26933</term> </sections> <topics> <term canonical="true">38029</term> <term>278</term> <term>50194</term> <term>63993</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">The Federal Trade Commission (FTC) voted Tuesday to ban noncompete agreements, possibly making it easier for doctors to switch employers without having to leave their communities and patients behind.</span> But business groups have vowed to challenge the decision in court.</p> <p>The <span class="Hyperlink"><a href="https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes">proposed final rule</a></span> passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.<br/><br/>Tensions around noncompetes have been building for years. In 2021, President Biden issued an <span class="Hyperlink"><a href="https://www.whitehouse.gov/briefing-room/presidential-actions/2021/07/09/executive-order-on-promoting-competition-in-the-american-economy/">executive order</a></span> supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/986904">proposed ending the restrictive covenants</a></span>.<br/><br/>While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.<br/><br/>US Chamber of Commerce president and CEO Suzanne P. Clark said in a <span class="Hyperlink"><a href="https://www.uschamber.com/finance/antitrust/u-s-chamber-to-sue-ftc-over-unlawful-power-grab-on-noncompete-agreements-ban">statement</a></span> that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.<br/><br/>The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.<br/><br/>Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/urologist-sues-health-system-over-noncompete-clause-2024a1000389">risk expensive litigation</a></span> for wanting to pursue job opportunities.<br/><br/>For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/hospital-mergers-2024-five-things-know-2024a100047m">hospital systems merging</a></span>, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/some-mds-long-covid-burnout-new-reality-2024a10006hq">significant burnout</a></span> that can shorten their [career] longevity.”<br/><br/>Commissioner Alvaro Bedoya said physicians have had their <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/989694">lives upended</a></span> by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.<br/><br/>It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.<br/><br/>“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the <span class="Hyperlink"><a href="https://www.congress.gov/bill/117th-congress/senate-bill/483">Workforce Mobility Act of 2021</a></span> and the <span class="Hyperlink"><a href="https://www.congress.gov/bill/118th-congress/senate-bill/379">Freedom to Compete Act of 2023</a></span>.<br/><br/>The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.<br/><br/></p> <h2>States, AMA Take Aim at Noncompetes</h2> <p>Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the <em>Journal of the American College of Cardiology</em>, <span class="Hyperlink"><a href="https://www.jacc.org/doi/10.1016/j.jacadv.2023.100547">12 states</a></span> prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.<br/><br/>The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in <span class="Hyperlink"><a href="https://www.oregon.gov/boli/employers/pages/noncompetition-agreements.aspx">Oregon</a></span>, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits <span class="Hyperlink"><a href="https://oag.dc.gov/blog/worker-alert-noncompete-provisions-are-now-illegal">2-year noncompetes</a></span> for “medical specialists” earning over $250,000 annually.<br/><br/>Indiana employers can no longer enter into noncompete agreements with <span class="Hyperlink"><a href="https://iga.in.gov/legislative/2023/bills/senate/7/details">primary care providers</a></span>. Other specialties may be subject to the clauses, except when the physician <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/994478">terminates the contract for cause</a></span> or when an employer terminates the contract without cause.<br/><br/>Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.<br/><br/>Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.<br/><br/>Although the American Medical Association (AMA) does not support a total ban, its House of Delegates <span class="Hyperlink"><a href="https://www.ama-assn.org/medical-residents/transition-resident-attending/ama-backs-effort-ban-many-physician-noncompete">adopted policies</a></span> last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.<br/><br/></p> <h2>Challenges Await</h2> <p>The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a <span class="Hyperlink"><a href="https://www.aha.org/press-releases/2024-04-23-aha-statement-final-ftc-noncompete-regulation">statement</a></span>.<br/><br/>To ease the transition to the new rule, the FTC also released a <span class="Hyperlink"><a href="https://www.ftc.gov/legal-library/browse/rules/noncompete-rule">model language</a></span> for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.<br/><br/>Dr. Marcus hopes the ban <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/are-you-ready-ai-be-better-doctor-than-you-2024a100070q">improves doctors’ lives</a></span>. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/federal-trade-commission-bans-noncompete-agreements-urges-2024a10007y0">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Are Women Better Doctors Than Men?
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.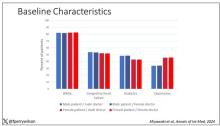
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 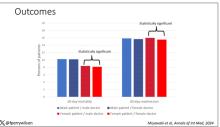
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167829</fileName> <TBEID>0C04FC71.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FC71</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T111623</QCDate> <firstPublished>20240424T113542</firstPublished> <LastPublished>20240424T113542</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T113541</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>F. Perry Wilson, MD</byline> <bylineText>F. PERRY WILSON, MD, MSCE</bylineText> <bylineFull>F. PERRY WILSON, MD, MSCE</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Female patients had a significantly lower 30-day mortality rate than male patients, but they fared even better when cared for by female doctors compared with ma</metaDescription> <articlePDF/> <teaserImage>301165</teaserImage> <teaser>Study finds female hospitalists provided better care, defined as lower 30-day mortality, than male hospitalists.</teaser> <title>Are Women Better Doctors Than Men?</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>card</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>cpn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>idprac</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdemed</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">21</term> <term>15</term> <term>5</term> <term>6</term> <term>34</term> <term>9</term> <term>13</term> <term>18</term> <term>20</term> <term>52226</term> <term>31</term> <term>22</term> <term>25</term> <term>23</term> <term>58877</term> <term>26</term> </publications> <sections> <term canonical="true">39313</term> </sections> <topics> <term canonical="true">38029</term> <term>278</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012878.jpg</altRep> <description role="drol:caption"/> <description role="drol:credit">Dr. Wilson</description> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012879.jpg</altRep> <description role="drol:caption"/> <description role="drol:credit">Dr. Wilson</description> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Are Women Better Doctors Than Men?</title> <deck/> </itemMeta> <itemContent> <p><br/><br/><em>This transcript has been edited for clarity</em>.</p> <p>It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?</p> <p>On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.<br/><br/>But this <span class="Hyperlink"><a href="https://www.acpjournals.org/doi/10.7326/M23-3163">study</a></span>, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in <em>Annals of Internal Medicine</em>, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.<br/><br/>In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.<br/><br/>Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.<br/><br/>The goal was to look at outcomes across four dyads:</p> <ul class="body"> <li>Male patient – male doctor</li> <li>Male patient – female doctor</li> <li>Female patient – male doctor</li> <li>Female patient – female doctor</li> </ul> <p>The primary outcome was 30-day mortality.<br/><br/>I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.<br/><br/>[[{"fid":"301165","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"Baseline characteristics","field_file_image_credit[und][0][value]":"Dr. Wilson","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_full_text"}}]]<br/><br/>Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.<br/><br/>So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.<br/><br/>I’ve graphed the results here. <span class="tag metaDescription">Female patients had a significantly lower 30-day mortality rate than male patients, but they fared even better when cared for by female doctors compared with male doctors. There wasn’t a particularly strong influence of physician sex on outcomes for male patients. The secondary outcome, 30-day hospital readmission, showed a similar trend.</span><br/><br/>[[{"fid":"301166","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"Outcomes","field_file_image_credit[und][0][value]":"Dr. Wilson","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_full_text"}}]]<br/><br/>This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.<br/><br/>So, what is going on here? I see four broad buckets of possibilities.<br/><br/>Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?<br/><br/>The authors cite <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3690315/">data that suggest</a></span> that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?<br/><br/>The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.<br/><br/>The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/647734">some prior studies have shown</a></span>. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.<br/><br/>Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?<br/><br/>And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.<br/><br/>Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.<span class="end"/></p> <p> <em>Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.</em> </p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/1000715">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>

