User login
Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167860</fileName> <TBEID>0C04FD2C.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FD2C</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240426T151917</QCDate> <firstPublished>20240426T152032</firstPublished> <LastPublished>20240426T152032</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240426T152032</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Deepa Varma</byline> <bylineText>DEEPA VARMA</bylineText> <bylineFull>DEEPA VARMA</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>A recent survey-based study found that only 4% of cancer survivors reported adhering to all four American Cancer Society (ACS) nutrition and physical activity g</metaDescription> <articlePDF/> <teaserImage/> <teaser>Researchers estimate more than 9,000 survivors’ adherence to weight, physical activity, fruit and vegetable consumption, and alcohol intake guidelines.</teaser> <title>Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>GIHOLD</publicationCode> <pubIssueName>January 2014</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term canonical="true">31</term> <term>18</term> <term>6</term> <term>15</term> <term>21</term> <term>23</term> <term>22</term> <term>13</term> </publications> <sections> <term canonical="true">27970</term> <term>39313</term> <term>86</term> </sections> <topics> <term>270</term> <term canonical="true">280</term> <term>198</term> <term>61821</term> <term>59244</term> <term>67020</term> <term>214</term> <term>217</term> <term>221</term> <term>238</term> <term>240</term> <term>242</term> <term>244</term> <term>39570</term> <term>245</term> <term>31848</term> <term>292</term> <term>178</term> <term>179</term> <term>181</term> <term>59374</term> <term>196</term> <term>197</term> <term>37637</term> <term>233</term> <term>243</term> <term>250</term> <term>49434</term> <term>303</term> <term>263</term> <term>192</term> <term>256</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines</title> <deck/> </itemMeta> <itemContent> <h2>TOPLINE:</h2> <p> <span class="tag metaDescription">A recent survey-based study found that only 4% of cancer survivors reported adhering to all four American Cancer Society (ACS) nutrition and physical activity guidelines, which include maintaining a healthy weight and diet, avoiding alcohol, and exercising regularly.</span> </p> <h2>METHODOLOGY:</h2> <ul class="body"> <li>The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.</li> <li>Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.</li> <li>The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.</li> <li>Overall, 9,121 survivors (91%) completed questionnaires for all four domains.</li> </ul> <h2>TAKEAWAY:</h2> <p>Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.<br/><br/>When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.<br/><br/>Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.<br/><br/>The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).</p> <h2>IN PRACTICE:</h2> <p>This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”</p> <h2>SOURCE:</h2> <p>This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamaoncology/fullarticle/2817661">online</a></span> in <em>JAMA Oncology</em>.</p> <h2>LIMITATIONS:</h2> <p>The authors reported several study limitations, most notably that self-reported data may introduce biases.</p> <h2>DISCLOSURES:</h2> <p>The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/few-cancer-survivors-meet-acs-nutrition-exercise-guidelines-2024a10007sl?src=">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Oregon Physician Assistants Get Name Change
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167861</fileName> <TBEID>0C04FD43.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FD43</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240426T111340</QCDate> <firstPublished>20240426T114737</firstPublished> <LastPublished>20240426T114737</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240426T114737</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Jennifer Nelson</byline> <bylineText>JENNIFER NELSON</bylineText> <bylineFull>JENNIFER NELSON</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>The switch is the first of its kind in the United States and comes on the heels of a decision from 2021 by the American Academy of Physician Associates (AAPA) t</metaDescription> <articlePDF/> <teaserImage/> <teaser>In June, Oregon PAs will be referred to as Physician Associates, a title change from Physician Assistants being debated nationwide. </teaser> <title>Oregon Physician Assistants Get Name Change</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>card</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>cpn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>idprac</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term>5</term> <term>6</term> <term>15</term> <term canonical="true">21</term> <term>9</term> <term>34</term> <term>13</term> <term>18</term> <term>20</term> <term>52226</term> <term>22</term> <term>23</term> <term>31</term> <term>25</term> <term>26</term> </publications> <sections> <term canonical="true">39313</term> </sections> <topics> <term canonical="true">38029</term> <term>278</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Oregon Physician Assistants Get Name Change</title> <deck/> </itemMeta> <itemContent> <p>On April 4, Oregon’s Governor Tina Kotek signed a <span class="Hyperlink"><a href="https://www.aapa.org/news-central/2024/04/oregon-governor-tina-kotek-signs-law-changing-pa-title/?utm_source=linkedin&utm_medium=aapa_post&utm_campaign=news_central">bill</a></span> into law that officially changed the title of “physician assistants” to “physician associates” in the state. <span class="tag metaDescription">The switch is the first of its kind in the United States and comes on the heels of a decision from 2021 by the American Academy of Physician Associates (AAPA) to change the meaning of “PA” to “physician associate” from “physician assistant.”</span></p> <p>In the <span class="Hyperlink"><a href="https://www.medscape.com/slideshow/2023-physician-assistant-satisfaction-6016503#2">Medscape Physician Assistant Career Satisfaction Report 2023</a>, </span>a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.<br/><br/>According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/985263">working independently</a></span> with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.<br/><br/>Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.<br/><br/>Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.<br/><br/>Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.<br/><br/>The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/oregon-physician-assistants-get-name-change-2024a100084h">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
‘We Need to Rethink Our Options’: Lung Cancer Recurrence
This transcript has been edited for clarity.
Hello. It’s Mark Kris reporting back after attending the New York Lung Cancer Foundation Summit here in New York. A large amount of discussion went on, but as usual, I was most interested in the perioperative space.
In previous videos, I’ve talked about this ongoing discussion of whether you should operate and give adjuvant therapy or give neoadjuvant therapy, and I’ve addressed that already. One thing I want to bring up – and as we move off of that argument, which frankly doesn’t have an answer today, with neoadjuvant therapy, having all the data to support it – is
I was taught early on by my surgical mentors that the issue here was systemic control. While they could do very successful surgery to get high levels of local control, they could not control systemic disease. Sadly, the tools we had early on with chemotherapy were just not good enough. Suddenly, we have better tools to control systemic spread. In the past, the vast majority of occurrences were systemic; they’re now local.
What I think we need to do as a group of practitioners trying to deal with the problems getting in the way of curing our patients is look at what the issue is now. Frankly, the big issue now, as systemic therapy has controlled metastatic disease, is recurrence in the chest.
We give adjuvant osimertinib. Please remember what the numbers are. In the osimertinib arm, of the 11 recurrences reported in the European Society for Medical Oncology presentation a few years back, nine of them were in the chest or mediastinal nodes. In the arm that got no osimertinib afterward, there were 46 recurrences, and 32 of those 46 recurrences were in the chest, either the lung or mediastinal nodes. Therefore, 74% of the recurrences are suddenly in the chest. What’s the issue here?
The issue is we need to find strategies to give better disease control in the chest, as we have made inroads in controlling systemic disease with the targeted therapies in the endothelial growth factor receptor space, and very likely the checkpoint inhibitors, too, as that data kind of filters out. We need to think about how better to get local control.
I think rather than continue to get into this argument of neoadjuvant vs adjuvant, we should move to what’s really hurting our patients. Again, the data I quoted you was from the ADAURA trial, which was adjuvant therapy, and I’m sure the neoadjuvant is going to show the same thing. It’s better systemic therapy but now, more trouble in the chest.
How are we going to deal with that? I’d like to throw out one strategy, and that is to rethink the role of radiation in these patients. Again, if the problem is local in the chest, lung, and lymph nodes, we have to think about local therapy. Yes, we’re not recommending it routinely for everybody, but now that we have better systemic control, we need to rethink our options. The obvious one to rethink is about giving radiotherapy.
We should also use what we learned in the earlier trials, which is that there is harm in giving excessive radiation to the heart. If you avoid the heart, you avoid the harm. We have better planning strategies for stereotactic body radiotherapy and more traditional radiation, and of course, we have proton therapy as well.
As we continue to struggle with the idea of that patient with stage II or III disease, whether to give adjuvant vs neoadjuvant therapy, please remember to consider their risk in 2024. Their risk for first recurrence is in the chest.
What are we going to do to better control disease in the chest? We have a challenge. I’m sure we can meet it if we put our heads together.
Dr. Kris is professor of medicine at Weill Cornell Medical College, and attending physician, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. It’s Mark Kris reporting back after attending the New York Lung Cancer Foundation Summit here in New York. A large amount of discussion went on, but as usual, I was most interested in the perioperative space.
In previous videos, I’ve talked about this ongoing discussion of whether you should operate and give adjuvant therapy or give neoadjuvant therapy, and I’ve addressed that already. One thing I want to bring up – and as we move off of that argument, which frankly doesn’t have an answer today, with neoadjuvant therapy, having all the data to support it – is
I was taught early on by my surgical mentors that the issue here was systemic control. While they could do very successful surgery to get high levels of local control, they could not control systemic disease. Sadly, the tools we had early on with chemotherapy were just not good enough. Suddenly, we have better tools to control systemic spread. In the past, the vast majority of occurrences were systemic; they’re now local.
What I think we need to do as a group of practitioners trying to deal with the problems getting in the way of curing our patients is look at what the issue is now. Frankly, the big issue now, as systemic therapy has controlled metastatic disease, is recurrence in the chest.
We give adjuvant osimertinib. Please remember what the numbers are. In the osimertinib arm, of the 11 recurrences reported in the European Society for Medical Oncology presentation a few years back, nine of them were in the chest or mediastinal nodes. In the arm that got no osimertinib afterward, there were 46 recurrences, and 32 of those 46 recurrences were in the chest, either the lung or mediastinal nodes. Therefore, 74% of the recurrences are suddenly in the chest. What’s the issue here?
The issue is we need to find strategies to give better disease control in the chest, as we have made inroads in controlling systemic disease with the targeted therapies in the endothelial growth factor receptor space, and very likely the checkpoint inhibitors, too, as that data kind of filters out. We need to think about how better to get local control.
I think rather than continue to get into this argument of neoadjuvant vs adjuvant, we should move to what’s really hurting our patients. Again, the data I quoted you was from the ADAURA trial, which was adjuvant therapy, and I’m sure the neoadjuvant is going to show the same thing. It’s better systemic therapy but now, more trouble in the chest.
How are we going to deal with that? I’d like to throw out one strategy, and that is to rethink the role of radiation in these patients. Again, if the problem is local in the chest, lung, and lymph nodes, we have to think about local therapy. Yes, we’re not recommending it routinely for everybody, but now that we have better systemic control, we need to rethink our options. The obvious one to rethink is about giving radiotherapy.
We should also use what we learned in the earlier trials, which is that there is harm in giving excessive radiation to the heart. If you avoid the heart, you avoid the harm. We have better planning strategies for stereotactic body radiotherapy and more traditional radiation, and of course, we have proton therapy as well.
As we continue to struggle with the idea of that patient with stage II or III disease, whether to give adjuvant vs neoadjuvant therapy, please remember to consider their risk in 2024. Their risk for first recurrence is in the chest.
What are we going to do to better control disease in the chest? We have a challenge. I’m sure we can meet it if we put our heads together.
Dr. Kris is professor of medicine at Weill Cornell Medical College, and attending physician, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. It’s Mark Kris reporting back after attending the New York Lung Cancer Foundation Summit here in New York. A large amount of discussion went on, but as usual, I was most interested in the perioperative space.
In previous videos, I’ve talked about this ongoing discussion of whether you should operate and give adjuvant therapy or give neoadjuvant therapy, and I’ve addressed that already. One thing I want to bring up – and as we move off of that argument, which frankly doesn’t have an answer today, with neoadjuvant therapy, having all the data to support it – is
I was taught early on by my surgical mentors that the issue here was systemic control. While they could do very successful surgery to get high levels of local control, they could not control systemic disease. Sadly, the tools we had early on with chemotherapy were just not good enough. Suddenly, we have better tools to control systemic spread. In the past, the vast majority of occurrences were systemic; they’re now local.
What I think we need to do as a group of practitioners trying to deal with the problems getting in the way of curing our patients is look at what the issue is now. Frankly, the big issue now, as systemic therapy has controlled metastatic disease, is recurrence in the chest.
We give adjuvant osimertinib. Please remember what the numbers are. In the osimertinib arm, of the 11 recurrences reported in the European Society for Medical Oncology presentation a few years back, nine of them were in the chest or mediastinal nodes. In the arm that got no osimertinib afterward, there were 46 recurrences, and 32 of those 46 recurrences were in the chest, either the lung or mediastinal nodes. Therefore, 74% of the recurrences are suddenly in the chest. What’s the issue here?
The issue is we need to find strategies to give better disease control in the chest, as we have made inroads in controlling systemic disease with the targeted therapies in the endothelial growth factor receptor space, and very likely the checkpoint inhibitors, too, as that data kind of filters out. We need to think about how better to get local control.
I think rather than continue to get into this argument of neoadjuvant vs adjuvant, we should move to what’s really hurting our patients. Again, the data I quoted you was from the ADAURA trial, which was adjuvant therapy, and I’m sure the neoadjuvant is going to show the same thing. It’s better systemic therapy but now, more trouble in the chest.
How are we going to deal with that? I’d like to throw out one strategy, and that is to rethink the role of radiation in these patients. Again, if the problem is local in the chest, lung, and lymph nodes, we have to think about local therapy. Yes, we’re not recommending it routinely for everybody, but now that we have better systemic control, we need to rethink our options. The obvious one to rethink is about giving radiotherapy.
We should also use what we learned in the earlier trials, which is that there is harm in giving excessive radiation to the heart. If you avoid the heart, you avoid the harm. We have better planning strategies for stereotactic body radiotherapy and more traditional radiation, and of course, we have proton therapy as well.
As we continue to struggle with the idea of that patient with stage II or III disease, whether to give adjuvant vs neoadjuvant therapy, please remember to consider their risk in 2024. Their risk for first recurrence is in the chest.
What are we going to do to better control disease in the chest? We have a challenge. I’m sure we can meet it if we put our heads together.
Dr. Kris is professor of medicine at Weill Cornell Medical College, and attending physician, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167859</fileName> <TBEID>0C04FD24.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FD24</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>353</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240425T173937</QCDate> <firstPublished>20240425T174806</firstPublished> <LastPublished>20240425T174806</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240425T174806</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Mark G. Kris, MD</byline> <bylineText>MARK G. KRIS, MD</bylineText> <bylineFull>MARK G. KRIS, MD</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>Opinion</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>what are the patterns of recurrence now that we have more successful systemic therapies, both targeted therapies and checkpoint inhibitors?</metaDescription> <articlePDF/> <teaserImage/> <teaser>“Suddenly, we have better tools to control systemic spread.”</teaser> <title>‘We Need to Rethink Our Options’: Lung Cancer Recurrence</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">31</term> <term>6</term> </publications> <sections> <term canonical="true">52</term> <term>41022</term> </sections> <topics> <term canonical="true">240</term> <term>270</term> <term>284</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>‘We Need to Rethink Our Options’: Lung Cancer Recurrence</title> <deck/> </itemMeta> <itemContent> <p><br/><br/><em>This transcript has been edited for clarity</em>.<br/><br/>Hello. It’s Mark Kris reporting back after attending the New York Lung Cancer Foundation Summit here in New York. A large amount of discussion went on, but as usual, I was most interested in the perioperative space.<br/><br/><span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/996828">In previous videos</a></span>, I’ve talked about this ongoing discussion of whether you should operate and give adjuvant therapy or give neoadjuvant therapy, and I’ve addressed that already. One thing I want to bring up – and as we move off of that argument, which frankly doesn’t have an answer today, with neoadjuvant therapy, having all the data to support it – is <span class="tag metaDescription">what are the patterns of recurrence now that we have more successful systemic therapies, both targeted therapies and checkpoint inhibitors?</span><br/><br/>I was taught early on by my surgical mentors that the issue here was systemic control. While they could do very successful surgery to get high levels of local control, they could not control systemic disease. Sadly, the tools we had early on with chemotherapy were just not good enough. Suddenly, we have better tools to control systemic spread. In the past, the vast majority of occurrences were systemic; they’re now local.<br/><br/>What I think we need to do as a group of practitioners trying to deal with the problems getting in the way of curing our patients is look at what the issue is now. Frankly, the big issue now, as systemic therapy has controlled metastatic disease, is recurrence in the chest.<br/><br/>We give adjuvant <span class="Hyperlink"><a href="https://reference.medscape.com/drug/tagrisso-osimertinib-1000062">osimertinib</a></span>. Please remember what the numbers are. In the osimertinib arm, of the 11 recurrences reported in the European Society for Medical Oncology presentation a few years back, nine of them were in the chest or mediastinal nodes. In the arm that got no osimertinib afterward, there were 46 recurrences, and 32 of those 46 recurrences were in the chest, either the lung or mediastinal nodes. Therefore, 74% of the recurrences are suddenly in the chest. What’s the issue here?<br/><br/>The issue is we need to find strategies to give better disease control in the chest, as we have made inroads in controlling systemic disease with the targeted therapies in the endothelial growth factor receptor space, and very likely the checkpoint inhibitors, too, as that data kind of filters out. We need to think about how better to get local control.<br/><br/>I think rather than continue to get into this argument of neoadjuvant vs adjuvant, we should move to what’s really hurting our patients. Again, the data I quoted you was from <span class="Hyperlink"><a href="https://clinicaltrials.gov/study/NCT02511106">the ADAURA trial</a></span>, which was adjuvant therapy, and I’m sure the neoadjuvant is going to show the same thing. It’s better systemic therapy but now, more trouble in the chest.<br/><br/>How are we going to deal with that? I’d like to throw out one strategy, and that is to rethink the role of radiation in these patients. Again, if the problem is local in the chest, lung, and lymph nodes, we have to think about local therapy. Yes, we’re not recommending it routinely for everybody, but now that we have better systemic control, we need to rethink our options. The obvious one to rethink is about giving radiotherapy.<br/><br/>We should also use what we learned in the earlier trials, which is that there is harm in giving excessive radiation to the heart. If you avoid the heart, you avoid the harm. We have better planning strategies for stereotactic body radiotherapy and more traditional radiation, and of course, we have proton therapy as well.<br/><br/>As we continue to struggle with the idea of that patient with stage II or III disease, whether to give adjuvant vs neoadjuvant therapy, please remember to consider their risk in 2024. Their risk for first recurrence is in the chest.<br/><br/>What are we going to do to better control disease in the chest? We have a challenge. I’m sure we can meet it if we put our heads together.<span class="end"/></p> <p> <em>Dr. Kris is professor of medicine at Weill Cornell Medical College, and attending physician, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.</em> </p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/1000627">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Can Rectal Cancer Patients Benefit from Deintensification of Treatment?
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167837</fileName> <TBEID>0C04FC82.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FC82</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>NCCN rectal cancer v4</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240425T151436</QCDate> <firstPublished>20240425T151455</firstPublished> <LastPublished>20240425T151455</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240425T151455</CMSDate> <articleSource>FROM NCCN 2024</articleSource> <facebookInfo/> <meetingNumber>5291-24</meetingNumber> <byline>Heidi Splete</byline> <bylineText>HEIDI SPLETE</bylineText> <bylineFull>HEIDI SPLETE</bylineFull> <bylineTitleText>MDedge News</bylineTitleText> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of </metaDescription> <articlePDF/> <teaserImage/> <teaser>An expert at the National Comprehensive Cancer Network annual conference discussed treatment-sparing strategies for eligible patients.</teaser> <title>Can Rectal Cancer Patients Benefit from Deintensification of Treatment?</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>GIHOLD</publicationCode> <pubIssueName>January 2014</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term canonical="true">31</term> </publications> <sections> <term>27980</term> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term>213</term> <term canonical="true">67020</term> <term>270</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Can Rectal Cancer Patients Benefit from Deintensification of Treatment?</title> <deck/> </itemMeta> <itemContent> <p>New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.</p> <p>Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said. <br/><br/>Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.</p> <p>However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.<br/><br/>During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer: </p> <ul class="body"> <li>Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.</li> <li>Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.</li> <li>Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery). </li> </ul> <h2>Does Watch and Wait Work?</h2> <p>Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.</p> <p>Data from the <span class="Hyperlink"><a href="https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.3520">OPRA</a></span> trial published in the <em>Journal of Clinical Oncology</em> showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said. <br/><br/></p> <h2> Does Selective Omission of Radiotherapy Work? </h2> <p>Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.</p> <p>Data from the <span class="Hyperlink"><a href="https://ascopubs.org/doi/10.1200/JCO.18.02309?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub 0pubmed">FOWARC trial,</a></span> published in the <em>Journal of Clinical Oncology</em> in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms: </p> <ul class="body"> <li>FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).</li> <li>FOLFOX plus chemoradiation. </li> <li>FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.</li> </ul> <p>Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.<br/><br/>The larger <span class="Hyperlink"><a href="https://www.nejm.org/doi/full/10.1056/NEJMoa2303269">PROSPECT study</a></span> published in <em>The New England Journal of Medicine</em> in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response. <br/><br/>“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said. <br/><br/>PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.<br/><br/></p> <h2> Does Dropping Everything But Immunotherapy Work? </h2> <p>Research is very preliminary, but a small <span class="Hyperlink"><a href="https://www.nejm.org/doi/full/10.1056/NEJMoa2201445">study</a></span> of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in <em>The New England Journal of Medicine</em> “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.</p> <p>This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”</p> <h2>What are the Clinical Implications and Next Steps? </h2> <p>Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.</p> <p>Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued. </p> <p>“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.<br/><br/>Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said. <br/><br/></p> <h2>What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?</h2> <p>The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.</p> <p>“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.<br/><br/>“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago. <br/><br/>The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.” <br/><br/>Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized. <br/><br/>The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said. <br/><br/>However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.<br/><br/>“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added. <br/><br/>“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.<br/><br/>Dr. Willett and Dr. Benson had no financial conflicts to disclose.</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM NCCN 2024
How These Young MDs Impressed the Hell Out of Their Bosses
Safe to say that anyone undertaking the physician journey does so with intense motivation and book smarts. Still, it can be incredibly hard to stand out. Everyone’s a go-getter, but what’s the X factor?
Here’s what they said ...
Lesson #1: Never Be Scared to Ask
Brien Barnewolt, MD, chairman and chief of the Department of Emergency Medicine at Tufts Medical Center, was very much surprised when a resident named Scott G. Weiner did something unexpected: Go after a job in the fall of his junior year residency instead of following the typical senior year trajectory.
“It’s very unusual for a trainee to apply for a job virtually a year ahead of schedule. But he knew what he wanted,” said Dr. Barnewolt. “I’d never had anybody come to me in that same scenario, and I’ve been doing this a long time.”
Under normal circumstances it would’ve been easy for Dr. Barnewolt to say no. But the unexpected request made him and his colleagues take a closer look, and they were impressed with Dr. Weiner’s skills. That, paired with his ambition and demeanor, compelled them to offer him an early job. But there’s more.
As the next year approached, Dr. Weiner explained he had an opportunity to work in emergency medicine in Tuscany and asked if he could take a 1-year delayed start for the position he applied a year early for.
The department held his position, and upon his return, Dr. Weiner made a lasting impact at Tufts before eventually moving on. “He outgrew us, which is nice to see,” Dr. Barnewolt said. (Dr. Weiner is currently McGraw Distinguished Chair in Emergency Medicine at Brigham and Women’s Hospital and associate professor at Harvard Medical School.)
Bottom line: Why did Dr. Barnewolt and his colleagues do so much to accommodate a young candidate? Yes, Dr. Weiner was talented, but he was also up-front about his ambitions from the get-go. Dr. Barnewolt said that kind of initiative can only be looked at positively.
“My advice would be, if you see an opportunity or a potential place where you might want to work, put out those feelers, start those conversations,” he said. “It’s not too early, especially in certain specialties, where the job market is very tight. Then, when circumstances change, be open about it and have that conversation. The worst that somebody can say is no, so it never hurts to be honest and open about where you want to go and what you want to be.”
Lesson #2: Chase Your Passion ‘Relentlessly’
Vance G. Fowler, MD, MHS, an infectious disease specialist at Duke University School of Medicine, runs a laboratory that researches methicillin-resistant Staphylococcus aureus (MRSA). Over the years, he’s mentored many doctors but understands the ambitions of young trainees don’t always align with the little free time that they have. “Many of them drop away when you give them a [side] project,” he said.
So when Tori Kinamon asked him to work on an MRSA project — in her first year — he gave her one that focused on researching vertebral osteomyelitis, a bone infection that can coincide with S aureus. What Dr. Fowler didn’t know: Kinamon (now MD) had been a competitive gymnast at Brown and battled her own life-threatening infection with MRSA.
“To my absolute astonishment, not only did she stick to it, but she was able to compile a presentation on the science and gave an oral presentation within a year of walking in the door,” said Dr. Fowler.
She went on to lead an initiative between the National Institutes of Health and US Food and Drug Administration to create endpoints for clinical drug trials, all of which occurred before starting her residency, which she’s about to embark upon.
Dr. Kinamon’s a good example, he said, of what happens when you add genuine passion to book smarts. Those who do always stand out because you can’t fake that. “Find your passion, and then chase it down relentlessly,” he said. “Once you’ve found your passion, things get easy because it stops being work and it starts being something else.”
If you haven’t identified a focus area, Dr. Fowler said to “be agnostic and observant. Keep your eyes open and your options open because you may surprise yourself. It may turn out that you end up liking something a whole lot more than you thought you did.”
Lesson #3: When You Say You’ve Always Wanted to Do Something, Do Something
As the chief of pulmonary and critical care medicine at the Northwestern Medicine Canning Thoracic Institute, Scott Budinger, MD, often hears lip service from doctors who want to put their skills to use in their local communities. One of his students actually did it.
Justin Fiala, MD, a pulmonary, critical care, and sleep specialist at Northwestern Medicine, joined Northwestern as a pulmonary fellow with a big interest in addressing health equity issues.
Dr. Fiala began volunteering with CommunityHealth during his fellowship and saw that many patients of the free Chicago-area clinic needed help with sleep disorders. He launched the organization’s first sleep clinic and its Patient-Centered Apnea Protocols Initiative.
“He developed a plan with some of the partners of the sleep apnea equipment to do home sleep testing for these patients that’s free of cost,” said Dr. Budinger.
Dr. Fiala goes in on Saturdays and runs a free clinic conducting sleep studies for patients and outfits them with devices that they need to improve their conditions, said Dr. Budinger.
“And these patients are the severest of the severe patients,” he said. “These are people that have severe sleep apnea that are driving around the roads, oftentimes don’t have insurance because they’re also precluded from having auto insurance. So, this is really something that not just benefits these patients but benefits our whole community.”
The fact that Dr. Fiala followed through on something that all doctors aspire to do — and in the middle of a very busy training program — is something that Dr. Budinger said makes him stand out in a big way.
“If you talk to any of our trainees or young faculty, everybody’s interested in addressing the issue of health disparities,” said Dr. Budinger. “Justin looked at that and said, ‘Well, you know, I’m not interested in talking about it. What can I do about this problem? And how can I actually get boots on the ground and help?’ That requires a big activation energy that many people don’t have.”
Lesson #4: Be a People-Person and a Patient-Person
When hiring employees at American Family Care in Portland, Oregon, Andrew Miller, MD, director of provider training, is always on the lookout for young MDs with emotional intelligence and a good bedside manner. He has been recently blown away, however, by a young physician’s assistant named Joseph Van Bindsbergen, PA-C, who was described as “all-around wonderful” during his reference check.
“Having less than 6 months of experience out of school, he is our highest ranked provider, whether it’s a nurse practitioner, PA, or doctor, in terms of patient satisfaction,” said Dr. Miller. The young PA has an “unprecedented perfect score” on his NPS rating.
Why? Patients said they’ve never felt as heard as they felt with Van Bindsbergen.
“That’s the thing I think that the up-and-coming providers should be focusing on is making your patients feel heard,” explained Dr. Miller. Van Bindsbergen is great at building rapport with a patient, whether they are 6 or 96. “He doesn’t just ask about sore throat symptoms. He asks, ‘what is the impact on your life of the sore throat? How does it affect your family or your work? What do you think this could be besides just strep? What are your concerns?’ ”
Dr. Miller said the magic of Van Bindsbergen is that he has an innate ability to look at patients “not just as a diagnosis but as a person, which they love.”
Lesson #5: Remember to Make That Difference With Each Patient
Doctors are used to swooping in and seeing a patient, ordering further testing if needed, and then moving on to the next patient. But one young intern at the start of his medical career broke this mold by giving a very anxious patient some much-needed support.
“There was a resident who was working overnight, and this poor young woman came in who had a new diagnosis of an advanced illness and a lot of anxiety around her condition, the newness of it, and the impact this is going to have on her family and her life,” said Elizabeth Horn Prsic, MD, assistant professor at Yale School of Medicine and firm chief for medical oncology and the director of Adult Inpatient Palliative Care.
Dr. Prsic found out the next morning that this trainee accompanied the patient to the MRI and held her hand as much as he was allowed to throughout the entire experience. “I was like, ‘wait you went down with her to radiology?’ And he’s like, ‘Yes, I was there the whole time,’ ” she recalled.
This gesture not only helped the patient feel calmer after receiving a potentially life-altering diagnosis but also helped ensure the test results were as clear as possible.
“If the study is not done well and a patient is moving or uncomfortable, it has to be stopped early or paused,” said Dr. Prsic. “Then the study is not very useful. In situations like these, medical decisions may be made based on imperfect data. The fact that we had this full complete good quality scan helped us get the care that she needed in a much timelier manner to help her and to move along the care that she that was medically appropriate for her.”
Dr. Prsic got emotional reflecting on the experience. Working at Yale, she saw a ton of intelligent doctors come through the ranks. But this gesture, she said, should serve as a reminder that “you don’t need to be the smartest person in the room to just be there for a patient. It was pure empathic presence and human connection. It gave me hope in the next generation of physicians.”
A version of this article appeared on Medscape.com.
Safe to say that anyone undertaking the physician journey does so with intense motivation and book smarts. Still, it can be incredibly hard to stand out. Everyone’s a go-getter, but what’s the X factor?
Here’s what they said ...
Lesson #1: Never Be Scared to Ask
Brien Barnewolt, MD, chairman and chief of the Department of Emergency Medicine at Tufts Medical Center, was very much surprised when a resident named Scott G. Weiner did something unexpected: Go after a job in the fall of his junior year residency instead of following the typical senior year trajectory.
“It’s very unusual for a trainee to apply for a job virtually a year ahead of schedule. But he knew what he wanted,” said Dr. Barnewolt. “I’d never had anybody come to me in that same scenario, and I’ve been doing this a long time.”
Under normal circumstances it would’ve been easy for Dr. Barnewolt to say no. But the unexpected request made him and his colleagues take a closer look, and they were impressed with Dr. Weiner’s skills. That, paired with his ambition and demeanor, compelled them to offer him an early job. But there’s more.
As the next year approached, Dr. Weiner explained he had an opportunity to work in emergency medicine in Tuscany and asked if he could take a 1-year delayed start for the position he applied a year early for.
The department held his position, and upon his return, Dr. Weiner made a lasting impact at Tufts before eventually moving on. “He outgrew us, which is nice to see,” Dr. Barnewolt said. (Dr. Weiner is currently McGraw Distinguished Chair in Emergency Medicine at Brigham and Women’s Hospital and associate professor at Harvard Medical School.)
Bottom line: Why did Dr. Barnewolt and his colleagues do so much to accommodate a young candidate? Yes, Dr. Weiner was talented, but he was also up-front about his ambitions from the get-go. Dr. Barnewolt said that kind of initiative can only be looked at positively.
“My advice would be, if you see an opportunity or a potential place where you might want to work, put out those feelers, start those conversations,” he said. “It’s not too early, especially in certain specialties, where the job market is very tight. Then, when circumstances change, be open about it and have that conversation. The worst that somebody can say is no, so it never hurts to be honest and open about where you want to go and what you want to be.”
Lesson #2: Chase Your Passion ‘Relentlessly’
Vance G. Fowler, MD, MHS, an infectious disease specialist at Duke University School of Medicine, runs a laboratory that researches methicillin-resistant Staphylococcus aureus (MRSA). Over the years, he’s mentored many doctors but understands the ambitions of young trainees don’t always align with the little free time that they have. “Many of them drop away when you give them a [side] project,” he said.
So when Tori Kinamon asked him to work on an MRSA project — in her first year — he gave her one that focused on researching vertebral osteomyelitis, a bone infection that can coincide with S aureus. What Dr. Fowler didn’t know: Kinamon (now MD) had been a competitive gymnast at Brown and battled her own life-threatening infection with MRSA.
“To my absolute astonishment, not only did she stick to it, but she was able to compile a presentation on the science and gave an oral presentation within a year of walking in the door,” said Dr. Fowler.
She went on to lead an initiative between the National Institutes of Health and US Food and Drug Administration to create endpoints for clinical drug trials, all of which occurred before starting her residency, which she’s about to embark upon.
Dr. Kinamon’s a good example, he said, of what happens when you add genuine passion to book smarts. Those who do always stand out because you can’t fake that. “Find your passion, and then chase it down relentlessly,” he said. “Once you’ve found your passion, things get easy because it stops being work and it starts being something else.”
If you haven’t identified a focus area, Dr. Fowler said to “be agnostic and observant. Keep your eyes open and your options open because you may surprise yourself. It may turn out that you end up liking something a whole lot more than you thought you did.”
Lesson #3: When You Say You’ve Always Wanted to Do Something, Do Something
As the chief of pulmonary and critical care medicine at the Northwestern Medicine Canning Thoracic Institute, Scott Budinger, MD, often hears lip service from doctors who want to put their skills to use in their local communities. One of his students actually did it.
Justin Fiala, MD, a pulmonary, critical care, and sleep specialist at Northwestern Medicine, joined Northwestern as a pulmonary fellow with a big interest in addressing health equity issues.
Dr. Fiala began volunteering with CommunityHealth during his fellowship and saw that many patients of the free Chicago-area clinic needed help with sleep disorders. He launched the organization’s first sleep clinic and its Patient-Centered Apnea Protocols Initiative.
“He developed a plan with some of the partners of the sleep apnea equipment to do home sleep testing for these patients that’s free of cost,” said Dr. Budinger.
Dr. Fiala goes in on Saturdays and runs a free clinic conducting sleep studies for patients and outfits them with devices that they need to improve their conditions, said Dr. Budinger.
“And these patients are the severest of the severe patients,” he said. “These are people that have severe sleep apnea that are driving around the roads, oftentimes don’t have insurance because they’re also precluded from having auto insurance. So, this is really something that not just benefits these patients but benefits our whole community.”
The fact that Dr. Fiala followed through on something that all doctors aspire to do — and in the middle of a very busy training program — is something that Dr. Budinger said makes him stand out in a big way.
“If you talk to any of our trainees or young faculty, everybody’s interested in addressing the issue of health disparities,” said Dr. Budinger. “Justin looked at that and said, ‘Well, you know, I’m not interested in talking about it. What can I do about this problem? And how can I actually get boots on the ground and help?’ That requires a big activation energy that many people don’t have.”
Lesson #4: Be a People-Person and a Patient-Person
When hiring employees at American Family Care in Portland, Oregon, Andrew Miller, MD, director of provider training, is always on the lookout for young MDs with emotional intelligence and a good bedside manner. He has been recently blown away, however, by a young physician’s assistant named Joseph Van Bindsbergen, PA-C, who was described as “all-around wonderful” during his reference check.
“Having less than 6 months of experience out of school, he is our highest ranked provider, whether it’s a nurse practitioner, PA, or doctor, in terms of patient satisfaction,” said Dr. Miller. The young PA has an “unprecedented perfect score” on his NPS rating.
Why? Patients said they’ve never felt as heard as they felt with Van Bindsbergen.
“That’s the thing I think that the up-and-coming providers should be focusing on is making your patients feel heard,” explained Dr. Miller. Van Bindsbergen is great at building rapport with a patient, whether they are 6 or 96. “He doesn’t just ask about sore throat symptoms. He asks, ‘what is the impact on your life of the sore throat? How does it affect your family or your work? What do you think this could be besides just strep? What are your concerns?’ ”
Dr. Miller said the magic of Van Bindsbergen is that he has an innate ability to look at patients “not just as a diagnosis but as a person, which they love.”
Lesson #5: Remember to Make That Difference With Each Patient
Doctors are used to swooping in and seeing a patient, ordering further testing if needed, and then moving on to the next patient. But one young intern at the start of his medical career broke this mold by giving a very anxious patient some much-needed support.
“There was a resident who was working overnight, and this poor young woman came in who had a new diagnosis of an advanced illness and a lot of anxiety around her condition, the newness of it, and the impact this is going to have on her family and her life,” said Elizabeth Horn Prsic, MD, assistant professor at Yale School of Medicine and firm chief for medical oncology and the director of Adult Inpatient Palliative Care.
Dr. Prsic found out the next morning that this trainee accompanied the patient to the MRI and held her hand as much as he was allowed to throughout the entire experience. “I was like, ‘wait you went down with her to radiology?’ And he’s like, ‘Yes, I was there the whole time,’ ” she recalled.
This gesture not only helped the patient feel calmer after receiving a potentially life-altering diagnosis but also helped ensure the test results were as clear as possible.
“If the study is not done well and a patient is moving or uncomfortable, it has to be stopped early or paused,” said Dr. Prsic. “Then the study is not very useful. In situations like these, medical decisions may be made based on imperfect data. The fact that we had this full complete good quality scan helped us get the care that she needed in a much timelier manner to help her and to move along the care that she that was medically appropriate for her.”
Dr. Prsic got emotional reflecting on the experience. Working at Yale, she saw a ton of intelligent doctors come through the ranks. But this gesture, she said, should serve as a reminder that “you don’t need to be the smartest person in the room to just be there for a patient. It was pure empathic presence and human connection. It gave me hope in the next generation of physicians.”
A version of this article appeared on Medscape.com.
Safe to say that anyone undertaking the physician journey does so with intense motivation and book smarts. Still, it can be incredibly hard to stand out. Everyone’s a go-getter, but what’s the X factor?
Here’s what they said ...
Lesson #1: Never Be Scared to Ask
Brien Barnewolt, MD, chairman and chief of the Department of Emergency Medicine at Tufts Medical Center, was very much surprised when a resident named Scott G. Weiner did something unexpected: Go after a job in the fall of his junior year residency instead of following the typical senior year trajectory.
“It’s very unusual for a trainee to apply for a job virtually a year ahead of schedule. But he knew what he wanted,” said Dr. Barnewolt. “I’d never had anybody come to me in that same scenario, and I’ve been doing this a long time.”
Under normal circumstances it would’ve been easy for Dr. Barnewolt to say no. But the unexpected request made him and his colleagues take a closer look, and they were impressed with Dr. Weiner’s skills. That, paired with his ambition and demeanor, compelled them to offer him an early job. But there’s more.
As the next year approached, Dr. Weiner explained he had an opportunity to work in emergency medicine in Tuscany and asked if he could take a 1-year delayed start for the position he applied a year early for.
The department held his position, and upon his return, Dr. Weiner made a lasting impact at Tufts before eventually moving on. “He outgrew us, which is nice to see,” Dr. Barnewolt said. (Dr. Weiner is currently McGraw Distinguished Chair in Emergency Medicine at Brigham and Women’s Hospital and associate professor at Harvard Medical School.)
Bottom line: Why did Dr. Barnewolt and his colleagues do so much to accommodate a young candidate? Yes, Dr. Weiner was talented, but he was also up-front about his ambitions from the get-go. Dr. Barnewolt said that kind of initiative can only be looked at positively.
“My advice would be, if you see an opportunity or a potential place where you might want to work, put out those feelers, start those conversations,” he said. “It’s not too early, especially in certain specialties, where the job market is very tight. Then, when circumstances change, be open about it and have that conversation. The worst that somebody can say is no, so it never hurts to be honest and open about where you want to go and what you want to be.”
Lesson #2: Chase Your Passion ‘Relentlessly’
Vance G. Fowler, MD, MHS, an infectious disease specialist at Duke University School of Medicine, runs a laboratory that researches methicillin-resistant Staphylococcus aureus (MRSA). Over the years, he’s mentored many doctors but understands the ambitions of young trainees don’t always align with the little free time that they have. “Many of them drop away when you give them a [side] project,” he said.
So when Tori Kinamon asked him to work on an MRSA project — in her first year — he gave her one that focused on researching vertebral osteomyelitis, a bone infection that can coincide with S aureus. What Dr. Fowler didn’t know: Kinamon (now MD) had been a competitive gymnast at Brown and battled her own life-threatening infection with MRSA.
“To my absolute astonishment, not only did she stick to it, but she was able to compile a presentation on the science and gave an oral presentation within a year of walking in the door,” said Dr. Fowler.
She went on to lead an initiative between the National Institutes of Health and US Food and Drug Administration to create endpoints for clinical drug trials, all of which occurred before starting her residency, which she’s about to embark upon.
Dr. Kinamon’s a good example, he said, of what happens when you add genuine passion to book smarts. Those who do always stand out because you can’t fake that. “Find your passion, and then chase it down relentlessly,” he said. “Once you’ve found your passion, things get easy because it stops being work and it starts being something else.”
If you haven’t identified a focus area, Dr. Fowler said to “be agnostic and observant. Keep your eyes open and your options open because you may surprise yourself. It may turn out that you end up liking something a whole lot more than you thought you did.”
Lesson #3: When You Say You’ve Always Wanted to Do Something, Do Something
As the chief of pulmonary and critical care medicine at the Northwestern Medicine Canning Thoracic Institute, Scott Budinger, MD, often hears lip service from doctors who want to put their skills to use in their local communities. One of his students actually did it.
Justin Fiala, MD, a pulmonary, critical care, and sleep specialist at Northwestern Medicine, joined Northwestern as a pulmonary fellow with a big interest in addressing health equity issues.
Dr. Fiala began volunteering with CommunityHealth during his fellowship and saw that many patients of the free Chicago-area clinic needed help with sleep disorders. He launched the organization’s first sleep clinic and its Patient-Centered Apnea Protocols Initiative.
“He developed a plan with some of the partners of the sleep apnea equipment to do home sleep testing for these patients that’s free of cost,” said Dr. Budinger.
Dr. Fiala goes in on Saturdays and runs a free clinic conducting sleep studies for patients and outfits them with devices that they need to improve their conditions, said Dr. Budinger.
“And these patients are the severest of the severe patients,” he said. “These are people that have severe sleep apnea that are driving around the roads, oftentimes don’t have insurance because they’re also precluded from having auto insurance. So, this is really something that not just benefits these patients but benefits our whole community.”
The fact that Dr. Fiala followed through on something that all doctors aspire to do — and in the middle of a very busy training program — is something that Dr. Budinger said makes him stand out in a big way.
“If you talk to any of our trainees or young faculty, everybody’s interested in addressing the issue of health disparities,” said Dr. Budinger. “Justin looked at that and said, ‘Well, you know, I’m not interested in talking about it. What can I do about this problem? And how can I actually get boots on the ground and help?’ That requires a big activation energy that many people don’t have.”
Lesson #4: Be a People-Person and a Patient-Person
When hiring employees at American Family Care in Portland, Oregon, Andrew Miller, MD, director of provider training, is always on the lookout for young MDs with emotional intelligence and a good bedside manner. He has been recently blown away, however, by a young physician’s assistant named Joseph Van Bindsbergen, PA-C, who was described as “all-around wonderful” during his reference check.
“Having less than 6 months of experience out of school, he is our highest ranked provider, whether it’s a nurse practitioner, PA, or doctor, in terms of patient satisfaction,” said Dr. Miller. The young PA has an “unprecedented perfect score” on his NPS rating.
Why? Patients said they’ve never felt as heard as they felt with Van Bindsbergen.
“That’s the thing I think that the up-and-coming providers should be focusing on is making your patients feel heard,” explained Dr. Miller. Van Bindsbergen is great at building rapport with a patient, whether they are 6 or 96. “He doesn’t just ask about sore throat symptoms. He asks, ‘what is the impact on your life of the sore throat? How does it affect your family or your work? What do you think this could be besides just strep? What are your concerns?’ ”
Dr. Miller said the magic of Van Bindsbergen is that he has an innate ability to look at patients “not just as a diagnosis but as a person, which they love.”
Lesson #5: Remember to Make That Difference With Each Patient
Doctors are used to swooping in and seeing a patient, ordering further testing if needed, and then moving on to the next patient. But one young intern at the start of his medical career broke this mold by giving a very anxious patient some much-needed support.
“There was a resident who was working overnight, and this poor young woman came in who had a new diagnosis of an advanced illness and a lot of anxiety around her condition, the newness of it, and the impact this is going to have on her family and her life,” said Elizabeth Horn Prsic, MD, assistant professor at Yale School of Medicine and firm chief for medical oncology and the director of Adult Inpatient Palliative Care.
Dr. Prsic found out the next morning that this trainee accompanied the patient to the MRI and held her hand as much as he was allowed to throughout the entire experience. “I was like, ‘wait you went down with her to radiology?’ And he’s like, ‘Yes, I was there the whole time,’ ” she recalled.
This gesture not only helped the patient feel calmer after receiving a potentially life-altering diagnosis but also helped ensure the test results were as clear as possible.
“If the study is not done well and a patient is moving or uncomfortable, it has to be stopped early or paused,” said Dr. Prsic. “Then the study is not very useful. In situations like these, medical decisions may be made based on imperfect data. The fact that we had this full complete good quality scan helped us get the care that she needed in a much timelier manner to help her and to move along the care that she that was medically appropriate for her.”
Dr. Prsic got emotional reflecting on the experience. Working at Yale, she saw a ton of intelligent doctors come through the ranks. But this gesture, she said, should serve as a reminder that “you don’t need to be the smartest person in the room to just be there for a patient. It was pure empathic presence and human connection. It gave me hope in the next generation of physicians.”
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167845</fileName> <TBEID>0C04FCA7.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FCA7</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T155925</QCDate> <firstPublished>20240424T155933</firstPublished> <LastPublished>20240424T155933</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T155933</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Nicole Pajer</byline> <bylineText>NICOLE PAJER</bylineText> <bylineFull>NICOLE PAJER</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>We asked five veteran doctors who have supervised scores of young medical professionals over the years to tell us about that one person who impressed the hell o</metaDescription> <articlePDF/> <teaserImage/> <teaser>Young doctors who got noticed shared their goals, followed through on their career passions, and made patients feel heard.</teaser> <title>How These Young MDs Impressed the Hell Out of Their Bosses</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>idprac</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>card</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term>6</term> <term canonical="true">21</term> <term>20</term> <term>15</term> <term>31</term> <term>25</term> <term>23</term> <term>52226</term> <term>18</term> <term>13</term> <term>34</term> <term>5</term> </publications> <sections> <term canonical="true">39313</term> </sections> <topics> <term canonical="true">38029</term> <term>278</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>How These Young MDs Impressed the Hell Out of Their Bosses</title> <deck/> </itemMeta> <itemContent> <p>Safe to say that anyone undertaking the physician journey does so with intense motivation and book smarts. Still, it can be incredibly hard to stand out. Everyone’s a go-getter, but what’s the X factor?</p> <p><span class="tag metaDescription">We asked five veteran doctors who have supervised scores of young medical professionals over the years to tell us about that one person who impressed the hell out of them — what they did, why it made them game changers, and what every doctor can learn from them.</span> Here’s what they said ...<br/><br/></p> <h2>Lesson #1: Never Be Scared to Ask</h2> <p><span class="Hyperlink"><a href="https://www.tuftsmedicine.org/doctor/brien-barnewolt">Brien Barnewolt, MD</a></span>, chairman and chief of the Department of Emergency Medicine at Tufts Medical Center, was very much surprised when a resident named Scott G. Weiner did something unexpected: Go after a job in the fall of his junior year residency instead of following the typical senior year trajectory.<br/><br/>“It’s very unusual for a trainee to apply for a job virtually a year ahead of schedule. But he knew what he wanted,” said Dr. Barnewolt. “I’d never had anybody come to me in that same scenario, and I’ve been doing this a long time.”<br/><br/>Under normal circumstances it would’ve been easy for Dr. Barnewolt to say no. But the unexpected request made him and his colleagues take a closer look, and they were impressed with Dr. Weiner’s skills. That, paired with his ambition and demeanor, compelled them to offer him an early job. But there’s more.<br/><br/>As the next year approached, Dr. Weiner explained he had an opportunity to work in emergency medicine in Tuscany and asked if he could take a 1-year delayed start for the position he applied a year early for.<br/><br/>The department held his position, and upon his return, Dr. Weiner made a lasting impact at Tufts before eventually moving on. “He outgrew us, which is nice to see,” Dr. Barnewolt said. (<span class="Hyperlink"><a href="https://physiciandirectory.brighamandwomens.org/details/12465/scott-weiner-emergency_medicine-boston">Dr. Weiner is currently</a></span> McGraw Distinguished Chair in Emergency Medicine at Brigham and Women’s Hospital and associate professor at Harvard Medical School.)<br/><br/>Bottom line: Why did Dr. Barnewolt and his colleagues do so much to accommodate a young candidate? Yes, Dr. Weiner was talented, but he was also up-front about his ambitions from the get-go. Dr. Barnewolt said that kind of initiative can only be looked at positively.<br/><br/>“My advice would be, if you see an opportunity or a potential place where you might want to work, put out those feelers, start those conversations,” he said. “It’s not too early, especially in certain specialties, where the job market is very tight. Then, when circumstances change, be open about it and have that conversation. The worst that somebody can say is no, so it never hurts to be honest and open about where you want to go and what you want to be.”<br/><br/></p> <h2>Lesson #2: Chase Your Passion ‘Relentlessly’</h2> <p><span class="Hyperlink"><a href="https://medicine.duke.edu/profile/vance-garrison-fowler">Vance G. Fowler, MD, MHS</a></span>, an infectious disease specialist at Duke University School of Medicine, runs a laboratory that researches methicillin-resistant <em>Staphylococcus aureus</em> (MRSA). Over the years, he’s mentored many doctors but understands the ambitions of young trainees don’t always align with the little free time that they have. “Many of them drop away when you give them a [side] project,” he said.<br/><br/>So when <span class="Hyperlink"><a href="https://medschool.duke.edu/stories/fighting-chance-against-infection">Tori Kinamon</a></span> asked him to work on an MRSA project — in her first year — he gave her one that focused on researching vertebral <span class="Hyperlink">osteomyelitis</span>, a bone infection that can coincide with <em>S aureus</em>. What Dr. Fowler didn’t know: Kinamon (now MD) had been a competitive gymnast at Brown and battled her own life-threatening infection with MRSA.<br/><br/>“To my absolute astonishment, not only did she stick to it, but she was able to compile a presentation on the science and gave an oral presentation within a year of walking in the door,” said Dr. Fowler.<br/><br/>She went on to lead an initiative between the National Institutes of Health and US Food and Drug Administration to create endpoints for clinical drug trials, all of which occurred before starting her residency, which she’s about to embark upon.<br/><br/>Dr. Kinamon’s a good example, he said, of what happens when you add genuine passion to book smarts. Those who do always stand out because you can’t fake that. “Find your passion, and then chase it down relentlessly,” he said. “Once you’ve found your passion, things get easy because it stops being work and it starts being something else.”<br/><br/>If you haven’t identified a focus area, Dr. Fowler said to “be agnostic and observant. Keep your eyes open and your options open because you may surprise yourself. It may turn out that you end up liking something a whole lot more than you thought you did.”<br/><br/></p> <h2>Lesson #3: When You Say You’ve Always Wanted to Do Something, Do Something</h2> <p>As the chief of pulmonary and critical care medicine at the Northwestern Medicine Canning Thoracic Institute, <span class="Hyperlink"><a href="https://www.feinberg.northwestern.edu/faculty-profiles/az/profile.html?xid=10309">Scott Budinger, MD,</a></span> often hears lip service from doctors who want to put their skills to use in their local communities. One of his students actually did it. <br/><br/><span class="Hyperlink"><a href="https://www.nm.org/doctors/1760821474/justin-anthony-fiala-md">Justin Fiala, MD</a></span>, a pulmonary, critical care, and sleep specialist at Northwestern Medicine, joined Northwestern as a pulmonary fellow with a big interest in addressing health equity issues.<br/><br/>Dr. Fiala began volunteering with CommunityHealth during his fellowship and saw that many patients of the free Chicago-area clinic needed help with sleep disorders. He launched the organization’s first sleep clinic and its Patient-Centered Apnea Protocols Initiative.<br/><br/>“He developed a plan with some of the partners of the sleep apnea equipment to do home sleep testing for these patients that’s free of cost,” said Dr. Budinger.<br/><br/>Dr. Fiala goes in on Saturdays and runs a free clinic conducting sleep studies for patients and outfits them with devices that they need to improve their conditions, said Dr. Budinger.<br/><br/>“And these patients are the severest of the severe patients,” he said. “These are people that have severe sleep apnea that are driving around the roads, oftentimes don’t have insurance because they’re also precluded from having auto insurance. So, this is really something that not just benefits these patients but benefits our whole community.”<br/><br/>The fact that Dr. Fiala followed through on something that all doctors aspire to do — and in the middle of a very busy training program — is something that Dr. Budinger said makes him stand out in a big way.<br/><br/>“If you talk to any of our trainees or young faculty, everybody’s interested in addressing the issue of health disparities,” said Dr. Budinger. “Justin looked at that and said, ‘Well, you know, I’m not interested in talking about it. What can I do about this problem? And how can I actually get boots on the ground and help?’ That requires a big activation energy that many people don’t have.”<br/><br/></p> <h2>Lesson #4: Be a People-Person and a Patient-Person</h2> <p>When hiring employees at American Family Care in Portland, Oregon, Andrew Miller, MD, director of provider training, is always on the lookout for young MDs with emotional intelligence and a good bedside manner. He has been recently blown away, however, by a young physician’s assistant named Joseph Van Bindsbergen, PA-C, who was described as “all-around wonderful” during his reference check.<br/><br/>“Having less than 6 months of experience out of school, he is our highest ranked provider, whether it’s a nurse practitioner, PA, or doctor, in terms of patient satisfaction,” said Dr. Miller. The young PA has an “unprecedented perfect score” on his NPS rating.<br/><br/>Why? Patients said they’ve never felt as heard as they felt with Van Bindsbergen.<br/><br/>“That’s the thing I think that the up-and-coming providers should be focusing on is making your patients feel heard,” explained Dr. Miller. Van Bindsbergen is great at building rapport with a patient, whether they are 6 or 96. “He doesn’t just ask about sore throat symptoms. He asks, ‘what is the impact on your life of the sore throat? How does it affect your family or your work? What do you think this could be besides just strep? What are your concerns?’ ”<br/><br/>Dr. Miller said the magic of Van Bindsbergen is that he has an innate ability to look at patients “not just as a diagnosis but as a person, which they love.”<br/><br/></p> <h2>Lesson #5: Remember to Make That Difference With Each Patient</h2> <p>Doctors are used to swooping in and seeing a patient, ordering further testing if needed, and then moving on to the next patient. But one young intern at the start of his medical career broke this mold by giving a very anxious patient some much-needed support.<br/><br/>“There was a resident who was working overnight, and this poor young woman came in who had a new diagnosis of an advanced illness and a lot of anxiety around her condition, the newness of it, and the impact this is going to have on her family and her life,” said <span class="Hyperlink"><a href="https://medicine.yale.edu/profile/elizabeth-prsic/">Elizabeth Horn Prsic, MD</a></span>, assistant professor at Yale School of Medicine and firm chief for medical oncology and the director of Adult Inpatient Palliative Care.<br/><br/>Dr. Prsic found out the next morning that this trainee accompanied the patient to the MRI and held her hand as much as he was allowed to throughout the entire experience. “I was like, ‘wait you went down with her to radiology?’ And he’s like, ‘Yes, I was there the whole time,’ ” she recalled.<br/><br/>This gesture not only helped the patient feel calmer after receiving a potentially life-altering diagnosis but also helped ensure the test results were as clear as possible.<br/><br/>“If the study is not done well and a patient is moving or uncomfortable, it has to be stopped early or paused,” said Dr. Prsic. “Then the study is not very useful. In situations like these, medical decisions may be made based on imperfect data. The fact that we had this full complete good quality scan helped us get the care that she needed in a much timelier manner to help her and to move along the care that she that was medically appropriate for her.”<br/><br/>Dr. Prsic got emotional reflecting on the experience. Working at Yale, she saw a ton of intelligent doctors come through the ranks. But this gesture, she said, should serve as a reminder that “you don’t need to be the smartest person in the room to just be there for a patient. It was pure empathic presence and human connection. It gave me hope in the next generation of physicians.”<br/><br/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/how-these-young-mds-impressed-hell-out-their-bosses-2024a10007wr">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167842</fileName> <TBEID>0C04FC9A.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FC9A</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T122750</QCDate> <firstPublished>20240424T122826</firstPublished> <LastPublished>20240424T122826</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T122826</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Steph Weber</byline> <bylineText>STEPH WEBER</bylineText> <bylineFull>STEPH WEBER</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>Feature</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>The Federal Trade Commission (FTC) voted Tuesday to ban noncompete agreements, possibly making it easier for doctors to switch employers without having to leave</metaDescription> <articlePDF/> <teaserImage/> <teaser>But dissenting commissioners dispute the FTC’s authority to broadly ban noncompetes.</teaser> <title>Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>card</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>cpn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>idprac</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>icymicov</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdemed</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> <publicationData> <publicationCode>mdid</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>GIHOLD</publicationCode> <pubIssueName>January 2014</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term>5</term> <term>34</term> <term>6</term> <term>9</term> <term>13</term> <term canonical="true">15</term> <term>18</term> <term>20</term> <term>21</term> <term>26</term> <term>31</term> <term>23</term> <term>25</term> <term>22</term> <term>52226</term> <term>69586</term> <term>58877</term> <term>51892</term> </publications> <sections> <term canonical="true">27980</term> <term>39313</term> <term>26933</term> </sections> <topics> <term canonical="true">38029</term> <term>278</term> <term>50194</term> <term>63993</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">The Federal Trade Commission (FTC) voted Tuesday to ban noncompete agreements, possibly making it easier for doctors to switch employers without having to leave their communities and patients behind.</span> But business groups have vowed to challenge the decision in court.</p> <p>The <span class="Hyperlink"><a href="https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes">proposed final rule</a></span> passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.<br/><br/>Tensions around noncompetes have been building for years. In 2021, President Biden issued an <span class="Hyperlink"><a href="https://www.whitehouse.gov/briefing-room/presidential-actions/2021/07/09/executive-order-on-promoting-competition-in-the-american-economy/">executive order</a></span> supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/986904">proposed ending the restrictive covenants</a></span>.<br/><br/>While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.<br/><br/>US Chamber of Commerce president and CEO Suzanne P. Clark said in a <span class="Hyperlink"><a href="https://www.uschamber.com/finance/antitrust/u-s-chamber-to-sue-ftc-over-unlawful-power-grab-on-noncompete-agreements-ban">statement</a></span> that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.<br/><br/>The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.<br/><br/>Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/urologist-sues-health-system-over-noncompete-clause-2024a1000389">risk expensive litigation</a></span> for wanting to pursue job opportunities.<br/><br/>For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/hospital-mergers-2024-five-things-know-2024a100047m">hospital systems merging</a></span>, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/some-mds-long-covid-burnout-new-reality-2024a10006hq">significant burnout</a></span> that can shorten their [career] longevity.”<br/><br/>Commissioner Alvaro Bedoya said physicians have had their <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/989694">lives upended</a></span> by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.<br/><br/>It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.<br/><br/>“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the <span class="Hyperlink"><a href="https://www.congress.gov/bill/117th-congress/senate-bill/483">Workforce Mobility Act of 2021</a></span> and the <span class="Hyperlink"><a href="https://www.congress.gov/bill/118th-congress/senate-bill/379">Freedom to Compete Act of 2023</a></span>.<br/><br/>The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.<br/><br/></p> <h2>States, AMA Take Aim at Noncompetes</h2> <p>Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the <em>Journal of the American College of Cardiology</em>, <span class="Hyperlink"><a href="https://www.jacc.org/doi/10.1016/j.jacadv.2023.100547">12 states</a></span> prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.<br/><br/>The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in <span class="Hyperlink"><a href="https://www.oregon.gov/boli/employers/pages/noncompetition-agreements.aspx">Oregon</a></span>, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits <span class="Hyperlink"><a href="https://oag.dc.gov/blog/worker-alert-noncompete-provisions-are-now-illegal">2-year noncompetes</a></span> for “medical specialists” earning over $250,000 annually.<br/><br/>Indiana employers can no longer enter into noncompete agreements with <span class="Hyperlink"><a href="https://iga.in.gov/legislative/2023/bills/senate/7/details">primary care providers</a></span>. Other specialties may be subject to the clauses, except when the physician <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/994478">terminates the contract for cause</a></span> or when an employer terminates the contract without cause.<br/><br/>Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.<br/><br/>Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.<br/><br/>Although the American Medical Association (AMA) does not support a total ban, its House of Delegates <span class="Hyperlink"><a href="https://www.ama-assn.org/medical-residents/transition-resident-attending/ama-backs-effort-ban-many-physician-noncompete">adopted policies</a></span> last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.<br/><br/></p> <h2>Challenges Await</h2> <p>The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a <span class="Hyperlink"><a href="https://www.aha.org/press-releases/2024-04-23-aha-statement-final-ftc-noncompete-regulation">statement</a></span>.<br/><br/>To ease the transition to the new rule, the FTC also released a <span class="Hyperlink"><a href="https://www.ftc.gov/legal-library/browse/rules/noncompete-rule">model language</a></span> for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.<br/><br/>Dr. Marcus hopes the ban <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/are-you-ready-ai-be-better-doctor-than-you-2024a100070q">improves doctors’ lives</a></span>. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/federal-trade-commission-bans-noncompete-agreements-urges-2024a10007y0">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Are Women Better Doctors Than Men?
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.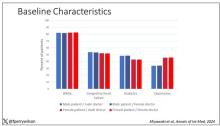
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 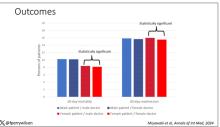
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167829</fileName> <TBEID>0C04FC71.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FC71</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T111623</QCDate> <firstPublished>20240424T113542</firstPublished> <LastPublished>20240424T113542</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T113541</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>F. Perry Wilson, MD</byline> <bylineText>F. PERRY WILSON, MD, MSCE</bylineText> <bylineFull>F. PERRY WILSON, MD, MSCE</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Female patients had a significantly lower 30-day mortality rate than male patients, but they fared even better when cared for by female doctors compared with ma</metaDescription> <articlePDF/> <teaserImage>301165</teaserImage> <teaser>Study finds female hospitalists provided better care, defined as lower 30-day mortality, than male hospitalists.</teaser> <title>Are Women Better Doctors Than Men?</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>card</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>cpn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>skin</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>hemn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>idprac</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>nr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle>Neurology Reviews</journalTitle> <journalFullTitle>Neurology Reviews</journalFullTitle> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>ob</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdemed</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">21</term> <term>15</term> <term>5</term> <term>6</term> <term>34</term> <term>9</term> <term>13</term> <term>18</term> <term>20</term> <term>52226</term> <term>31</term> <term>22</term> <term>25</term> <term>23</term> <term>58877</term> <term>26</term> </publications> <sections> <term canonical="true">39313</term> </sections> <topics> <term canonical="true">38029</term> <term>278</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012878.jpg</altRep> <description role="drol:caption"/> <description role="drol:credit">Dr. Wilson</description> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012879.jpg</altRep> <description role="drol:caption"/> <description role="drol:credit">Dr. Wilson</description> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Are Women Better Doctors Than Men?</title> <deck/> </itemMeta> <itemContent> <p><br/><br/><em>This transcript has been edited for clarity</em>.</p> <p>It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?</p> <p>On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.<br/><br/>But this <span class="Hyperlink"><a href="https://www.acpjournals.org/doi/10.7326/M23-3163">study</a></span>, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in <em>Annals of Internal Medicine</em>, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.<br/><br/>In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.<br/><br/>Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.<br/><br/>The goal was to look at outcomes across four dyads:</p> <ul class="body"> <li>Male patient – male doctor</li> <li>Male patient – female doctor</li> <li>Female patient – male doctor</li> <li>Female patient – female doctor</li> </ul> <p>The primary outcome was 30-day mortality.<br/><br/>I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.<br/><br/>[[{"fid":"301165","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"Baseline characteristics","field_file_image_credit[und][0][value]":"Dr. Wilson","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_full_text"}}]]<br/><br/>Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.<br/><br/>So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.<br/><br/>I’ve graphed the results here. <span class="tag metaDescription">Female patients had a significantly lower 30-day mortality rate than male patients, but they fared even better when cared for by female doctors compared with male doctors. There wasn’t a particularly strong influence of physician sex on outcomes for male patients. The secondary outcome, 30-day hospital readmission, showed a similar trend.</span><br/><br/>[[{"fid":"301166","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"Outcomes","field_file_image_credit[und][0][value]":"Dr. Wilson","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_full_text"}}]]<br/><br/>This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.<br/><br/>So, what is going on here? I see four broad buckets of possibilities.<br/><br/>Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?<br/><br/>The authors cite <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3690315/">data that suggest</a></span> that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?<br/><br/>The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.<br/><br/>The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/647734">some prior studies have shown</a></span>. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.<br/><br/>Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?<br/><br/>And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.<br/><br/>Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.<span class="end"/></p> <p> <em>Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.</em> </p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/1000715">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Is Osimertinib Better Alone or With Chemotherapy in Non–Small Cell Lung Cancer?
SAN DIEGO —
That is a question brewing among some oncologists now that the US Food and Drug Administration (FDA) has approved osimertinib (Tagrisso, AstraZeneca) for both indications in patients with epidermal growth factor receptor (EGFR) mutations.
An answer began to emerge in research presented at the American Association for Cancer Research annual meeting.
An exploratory analysis of the FLAURA2 trial found that, when patients have EGFR mutations on baseline circulating tumor DNA (ctDNA) testing, the combination treatment can extend progression-free survival (PFS). In this patient group, those receiving osimertinib alongside pemetrexed plus cisplatin or carboplatin had a 9-month PFS advantage compared with those who received osimertinib alone.
Conversely, when patients do not have EGFR mutations following baseline ctDNA testing, osimertinib alone appears to offer similar PFS outcomes to the combination therapy, but with less toxicity.
“Baseline detection of plasma EGFR mutations may identify a subgroup of patients who derive most benefit from the addition of platinum-pemetrexed to osimertinib as first-line treatment of EGFR-mutated advance non–small cell lung cancer,” investigator Pasi A. Jänne, MD, PhD, a lung cancer oncologist at the Dana-Farber Cancer Institute, Boston, said during his presentation.
The FLAURA2 trial randomized 557 patients equally to daily osimertinib either alone or with pemetrexed plus cisplatin or carboplatin every 3 weeks for four cycles followed by pemetrexed every 3 weeks until disease progression or unacceptable toxicity.
Patients were tested for Ex19del or L858R EGFR mutations at baseline and at 3 and 6 weeks; baseline mutations were found in 73% of evaluable patients.
In patients with baseline mutations, the median PFS was 24.8 months with the combination therapy vs 13.9 months with osimertinib alone (hazard ratio [HR], 0.60).
In patients without baseline mutations, the median PFS was similar in both groups — 33.3 months with the combination vs 30.3 months with monotherapy (HR, 0.93; 95% CI, 0.51-1.72).
The investigators also found that having baseline mutations was associated with worse outcomes regardless of study arm, and mutation clearance was associated with improved outcomes. Clearance occurred more quickly among patients receiving the combination treatment, but almost 90% of patients in both arms cleared their mutations by week 6.
“As we move forward and think about which of our patients we would treat with the combination ... the presence of baseline EGFR mutations in ctDNA may be one of the features that goes into the conversation,” Dr. Jänne said.
Study discussant Marina Chiara Garassino, MD, a thoracic oncologist at the University of Chicago, agreed that this trial can help oncologists make this kind of treatment decision.
Patients with baseline EGFR mutations also tended to have larger tumors, more brain metastases, and worse performance scores; the combination therapy makes sense when such factors are present in patients with baseline EGFR mutations, Dr. Garassino said.
The wrinkle in the findings is that the study used digital droplet polymerase chain reaction (Biodesix) to test for EGFR mutations, which is not commonly used. Clinicians often use next-generation sequencing, which is less sensitive and can lead to false negatives.
It makes it difficult to know how to apply the findings to everyday practice, but Janne hopes a study will be done to correlate next-generation sequencing detection with outcomes.
The study was funded by AstraZeneca, maker of osimertinib, and researchers included AstraZeneca employees. Dr. Jänne is a consultant for and reported research funding from the company. He is a co-inventor on an EGFR mutations patent. Dr. Garassino is also an AstraZeneca consultant and reported institutional financial interests in the company.
A version of this article appeared on Medscape.com.
SAN DIEGO —
That is a question brewing among some oncologists now that the US Food and Drug Administration (FDA) has approved osimertinib (Tagrisso, AstraZeneca) for both indications in patients with epidermal growth factor receptor (EGFR) mutations.
An answer began to emerge in research presented at the American Association for Cancer Research annual meeting.
An exploratory analysis of the FLAURA2 trial found that, when patients have EGFR mutations on baseline circulating tumor DNA (ctDNA) testing, the combination treatment can extend progression-free survival (PFS). In this patient group, those receiving osimertinib alongside pemetrexed plus cisplatin or carboplatin had a 9-month PFS advantage compared with those who received osimertinib alone.
Conversely, when patients do not have EGFR mutations following baseline ctDNA testing, osimertinib alone appears to offer similar PFS outcomes to the combination therapy, but with less toxicity.
“Baseline detection of plasma EGFR mutations may identify a subgroup of patients who derive most benefit from the addition of platinum-pemetrexed to osimertinib as first-line treatment of EGFR-mutated advance non–small cell lung cancer,” investigator Pasi A. Jänne, MD, PhD, a lung cancer oncologist at the Dana-Farber Cancer Institute, Boston, said during his presentation.
The FLAURA2 trial randomized 557 patients equally to daily osimertinib either alone or with pemetrexed plus cisplatin or carboplatin every 3 weeks for four cycles followed by pemetrexed every 3 weeks until disease progression or unacceptable toxicity.
Patients were tested for Ex19del or L858R EGFR mutations at baseline and at 3 and 6 weeks; baseline mutations were found in 73% of evaluable patients.
In patients with baseline mutations, the median PFS was 24.8 months with the combination therapy vs 13.9 months with osimertinib alone (hazard ratio [HR], 0.60).
In patients without baseline mutations, the median PFS was similar in both groups — 33.3 months with the combination vs 30.3 months with monotherapy (HR, 0.93; 95% CI, 0.51-1.72).
The investigators also found that having baseline mutations was associated with worse outcomes regardless of study arm, and mutation clearance was associated with improved outcomes. Clearance occurred more quickly among patients receiving the combination treatment, but almost 90% of patients in both arms cleared their mutations by week 6.
“As we move forward and think about which of our patients we would treat with the combination ... the presence of baseline EGFR mutations in ctDNA may be one of the features that goes into the conversation,” Dr. Jänne said.
Study discussant Marina Chiara Garassino, MD, a thoracic oncologist at the University of Chicago, agreed that this trial can help oncologists make this kind of treatment decision.
Patients with baseline EGFR mutations also tended to have larger tumors, more brain metastases, and worse performance scores; the combination therapy makes sense when such factors are present in patients with baseline EGFR mutations, Dr. Garassino said.
The wrinkle in the findings is that the study used digital droplet polymerase chain reaction (Biodesix) to test for EGFR mutations, which is not commonly used. Clinicians often use next-generation sequencing, which is less sensitive and can lead to false negatives.
It makes it difficult to know how to apply the findings to everyday practice, but Janne hopes a study will be done to correlate next-generation sequencing detection with outcomes.
The study was funded by AstraZeneca, maker of osimertinib, and researchers included AstraZeneca employees. Dr. Jänne is a consultant for and reported research funding from the company. He is a co-inventor on an EGFR mutations patent. Dr. Garassino is also an AstraZeneca consultant and reported institutional financial interests in the company.
A version of this article appeared on Medscape.com.
SAN DIEGO —
That is a question brewing among some oncologists now that the US Food and Drug Administration (FDA) has approved osimertinib (Tagrisso, AstraZeneca) for both indications in patients with epidermal growth factor receptor (EGFR) mutations.
An answer began to emerge in research presented at the American Association for Cancer Research annual meeting.
An exploratory analysis of the FLAURA2 trial found that, when patients have EGFR mutations on baseline circulating tumor DNA (ctDNA) testing, the combination treatment can extend progression-free survival (PFS). In this patient group, those receiving osimertinib alongside pemetrexed plus cisplatin or carboplatin had a 9-month PFS advantage compared with those who received osimertinib alone.
Conversely, when patients do not have EGFR mutations following baseline ctDNA testing, osimertinib alone appears to offer similar PFS outcomes to the combination therapy, but with less toxicity.
“Baseline detection of plasma EGFR mutations may identify a subgroup of patients who derive most benefit from the addition of platinum-pemetrexed to osimertinib as first-line treatment of EGFR-mutated advance non–small cell lung cancer,” investigator Pasi A. Jänne, MD, PhD, a lung cancer oncologist at the Dana-Farber Cancer Institute, Boston, said during his presentation.
The FLAURA2 trial randomized 557 patients equally to daily osimertinib either alone or with pemetrexed plus cisplatin or carboplatin every 3 weeks for four cycles followed by pemetrexed every 3 weeks until disease progression or unacceptable toxicity.
Patients were tested for Ex19del or L858R EGFR mutations at baseline and at 3 and 6 weeks; baseline mutations were found in 73% of evaluable patients.
In patients with baseline mutations, the median PFS was 24.8 months with the combination therapy vs 13.9 months with osimertinib alone (hazard ratio [HR], 0.60).
In patients without baseline mutations, the median PFS was similar in both groups — 33.3 months with the combination vs 30.3 months with monotherapy (HR, 0.93; 95% CI, 0.51-1.72).
The investigators also found that having baseline mutations was associated with worse outcomes regardless of study arm, and mutation clearance was associated with improved outcomes. Clearance occurred more quickly among patients receiving the combination treatment, but almost 90% of patients in both arms cleared their mutations by week 6.
“As we move forward and think about which of our patients we would treat with the combination ... the presence of baseline EGFR mutations in ctDNA may be one of the features that goes into the conversation,” Dr. Jänne said.
Study discussant Marina Chiara Garassino, MD, a thoracic oncologist at the University of Chicago, agreed that this trial can help oncologists make this kind of treatment decision.
Patients with baseline EGFR mutations also tended to have larger tumors, more brain metastases, and worse performance scores; the combination therapy makes sense when such factors are present in patients with baseline EGFR mutations, Dr. Garassino said.
The wrinkle in the findings is that the study used digital droplet polymerase chain reaction (Biodesix) to test for EGFR mutations, which is not commonly used. Clinicians often use next-generation sequencing, which is less sensitive and can lead to false negatives.
It makes it difficult to know how to apply the findings to everyday practice, but Janne hopes a study will be done to correlate next-generation sequencing detection with outcomes.
The study was funded by AstraZeneca, maker of osimertinib, and researchers included AstraZeneca employees. Dr. Jänne is a consultant for and reported research funding from the company. He is a co-inventor on an EGFR mutations patent. Dr. Garassino is also an AstraZeneca consultant and reported institutional financial interests in the company.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167833</fileName> <TBEID>0C04FC79.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FC79</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T095456</QCDate> <firstPublished>20240424T095633</firstPublished> <LastPublished>20240424T095633</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T095633</CMSDate> <articleSource>FROM AACR</articleSource> <facebookInfo/> <meetingNumber>2976-24</meetingNumber> <byline>M Alex Otto</byline> <bylineText>M. ALEXANDER OTTO, PA, MMS</bylineText> <bylineFull>M. ALEXANDER OTTO, PA, MMS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>When should patients with advanced or metastatic non–small cell lung cancer receive osimertinib plus platinum-based chemotherapy in the frontline setting and wh</metaDescription> <articlePDF/> <teaserImage/> <teaser>Randomized trial of 557 patients compares taking osimertinib alone to osimertinib with pemetrexed plus cisplatin or carboplatin, followed by pemetrexed.</teaser> <title>Is Osimertinib Better Alone or With Chemotherapy in Non–Small Cell Lung Cancer?</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term>6</term> <term canonical="true">31</term> </publications> <sections> <term>39313</term> <term>27980</term> <term canonical="true">53</term> </sections> <topics> <term>284</term> <term canonical="true">240</term> <term>270</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Is Osimertinib Better Alone or With Chemotherapy in Non–Small Cell Lung Cancer?</title> <deck/> </itemMeta> <itemContent> <p>SAN DIEGO — <span class="tag metaDescription">When should patients with advanced or metastatic non–small cell lung cancer receive osimertinib plus platinum-based chemotherapy in the frontline setting and when is osimertinib enough on its own?</span></p> <p>That is a question brewing among some oncologists now that the US Food and Drug Administration (FDA) <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/osimertinib-plus-chemo-approved-egfr-mutated-nsclc-2024a10003j5">has approved</a></span> osimertinib (Tagrisso, AstraZeneca) for both indications in patients with epidermal growth factor receptor (EGFR) mutations.<br/><br/>An answer began to emerge in research presented at the <span class="Hyperlink"><a href="https://www.medscape.com/viewcollection/37452">American Association for Cancer Research annual meeting</a></span>.<br/><br/>An <span class="Hyperlink"><a href="https://www.abstractsonline.com/pp8/#!/20272/presentation/11432">exploratory analysis</a> </span>of the <span class="Hyperlink"><a href="https://pubmed.ncbi.nlm.nih.gov/37937763/">FLAURA2</a></span> trial found that, when patients have EGFR mutations on baseline circulating tumor DNA (ctDNA) testing, the combination treatment can extend progression-free survival (PFS). In this patient group, those receiving osimertinib alongside <span class="Hyperlink">pemetrexed</span> plus <span class="Hyperlink">cisplatin</span> or <span class="Hyperlink">carboplatin</span> had a 9-month PFS advantage compared with those who received osimertinib alone.<br/><br/>Conversely, when patients do not have EGFR mutations following baseline ctDNA testing, osimertinib alone appears to offer similar PFS outcomes to the combination therapy, but with less toxicity.<br/><br/>“Baseline detection of plasma EGFR mutations may identify a subgroup of patients who derive most benefit from the addition of platinum-pemetrexed to osimertinib as first-line treatment of EGFR-mutated advance non–small cell lung cancer,” investigator <a href="https://www.dana-farber.org/find-a-doctor/pasi-a-janne"><span class="Hyperlink">Pasi A. J</span><span class="Hyperlink">ä</span><span class="Hyperlink">nne</span></a>, MD, PhD, a lung cancer oncologist at the Dana-Farber Cancer Institute, Boston, said during his presentation.<br/><br/>The FLAURA2 trial randomized 557 patients equally to daily osimertinib either alone or with pemetrexed plus cisplatin or carboplatin every 3 weeks for four cycles followed by pemetrexed every 3 weeks until disease progression or unacceptable toxicity.<br/><br/>Patients were tested for Ex19del or L858R EGFR mutations at baseline and at 3 and 6 weeks; baseline mutations were found in 73% of evaluable patients.<br/><br/>In patients with baseline mutations, the median PFS was 24.8 months with the combination therapy vs 13.9 months with osimertinib alone (hazard ratio [HR], 0.60).<br/><br/>In patients without baseline mutations, the median PFS was similar in both groups — 33.3 months with the combination vs 30.3 months with monotherapy (HR, 0.93; 95% CI, 0.51-1.72).<br/><br/>The investigators also found that having baseline mutations was associated with worse outcomes regardless of study arm, and mutation clearance was associated with improved outcomes. Clearance occurred more quickly among patients receiving the combination treatment, but almost 90% of patients in both arms cleared their mutations by week 6.<br/><br/>“As we move forward and think about which of our patients we would treat with the combination ... the presence of baseline EGFR mutations in ctDNA may be one of the features that goes into the conversation,” Dr. J<span class="Hyperlink">ä</span>nne said.<br/><br/>Study discussant <span class="Hyperlink"><a href="https://www.uchicagomedicine.org/find-a-physician/physician/marina-chiara-garassino">Marina Chiara Garassino</a></span>, MD, a thoracic oncologist at the University of Chicago, agreed that this trial can help oncologists make this kind of treatment decision.<br/><br/>Patients with baseline EGFR mutations also tended to have larger tumors, more <span class="Hyperlink">brain metastases</span>, and worse performance scores; the combination therapy makes sense when such factors are present in patients with baseline EGFR mutations, Dr. Garassino said.<br/><br/>The wrinkle in the findings is that the study used digital droplet polymerase chain reaction (Biodesix) to test for EGFR mutations, which is not commonly used. Clinicians often use next-generation sequencing, which is less sensitive and can lead to false negatives.<br/><br/>It makes it difficult to know how to apply the findings to everyday practice, but Janne hopes a study will be done to correlate next-generation sequencing detection with outcomes.<br/><br/>The study was funded by AstraZeneca, maker of osimertinib, and researchers included AstraZeneca employees. Dr. J<span class="Hyperlink">ä</span>nne is a consultant for and reported research funding from the company. He is a co-inventor on an EGFR mutations patent. Dr. Garassino is also an AstraZeneca consultant and reported institutional financial interests in the company.<br/><br/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/osimertinib-better-alone-or-chemotherapy-nsclc-2024a10007sx">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM AACR
FDA Approves New Bladder Cancer Drug
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167832</fileName> <TBEID>0C04FC77.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FC77</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240424T091016</QCDate> <firstPublished>20240424T091038</firstPublished> <LastPublished>20240424T091038</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240424T091038</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>S Worcester</byline> <bylineText>SHARON WORCESTER, MA</bylineText> <bylineFull>SHARON WORCESTER, MA</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>The US Food and Drug Administration (FDA) has approved the first-in-class interleukin (IL)-15 superagonist nogapendekin alfa inbakicept-pmln (Anktiva), plus bac</metaDescription> <articlePDF/> <teaserImage/> <teaser>The FDA declined an initial approval for the combination in May 2023.</teaser> <title>FDA Approves New Bladder Cancer Drug</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">31</term> </publications> <sections> <term>39313</term> <term canonical="true">27979</term> <term>37225</term> <term>27980</term> </sections> <topics> <term>270</term> <term>278</term> <term canonical="true">214</term> <term>232</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>FDA Approves New Bladder Cancer Drug</title> <deck/> </itemMeta> <itemContent> <p> <span class="tag metaDescription">The US Food and Drug Administration (FDA) has approved the first-in-class interleukin (IL)-15 superagonist <span class="Hyperlink"><a href="https://reference.medscape.com/drug/anktiva-nonapendekine-alfa-4000332">nogapendekin alfa</a></span> inbakicept-pmln (Anktiva), plus bacillus Calmette-Guérin (BCG), for the treatment of certain non–muscle-invasive bladder cancers that fail to respond to BCG alone.</span> </p> <p>Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive <span class="Hyperlink">bladder cancer</span> carcinoma in situ with or without Ta or T1 papillary disease. <br/><br/>The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which <span class="Hyperlink"><a href="https://immunitybio.com/fda-accepts-immunitybios-bla-resubmission-as-complete-and-sets-new-pdufa-date/">was accepted</a></span>.<br/><br/>The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a <span class="Hyperlink"><a href="https://immunitybio.com/nejm-evidence-publishes-results-for-immunitybios-quilt-3-032-registrational-trial-of-il-15-superagonist-n-803-plus-bcg-in-patients-with-bladder-cancer/">press release</a></span> announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience. <br/><br/>Approval was based on findings from the single arm, phase 2/3 open-label <span class="Hyperlink"><a href="https://classic.clinicaltrials.gov/ct2/show/NCT03022825">QUILT-3.032 study</a></span>, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2. <br/><br/>Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months. <br/><br/>According to the <span class="Hyperlink"><a href="https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nogapendekin-alfa-inbakicept-pmln-bcg-unresponsive-non-muscle-invasive-bladder-cancer">FDA’s press release</a></span>, 62% of patients had a complete response, defined as a negative <span class="Hyperlink">cystoscopy</span> and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.<br/><br/>The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and <span class="Hyperlink">hematuria</span>, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.<br/><br/>The <span class="Hyperlink"><a href="https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761336s000lbl.pdf">recommended dose</a></span> is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.<br/><br/>The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity. <br/><br/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/fda-approves-new-bladder-cancer-drug-2024a10007t5">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
CRC Screening in Primary Care: The Blood Test Option
Last year, I concluded a commentary for this news organization on colorectal cancer (CRC) screening guidelines by stating that between stool-based tests, flexible sigmoidoscopy, and colonoscopy, “the best screening test is the test that gets done.” But should that maxim apply to the new blood-based screening test, Guardant Health Shield? This proprietary test, which costs $895 and is not generally covered by insurance, identifies alterations in cell-free DNA that are characteristic of CRC.
Shield’s test characteristics were recently evaluated in a prospective study of more than 10,000 adults aged 45-84 at average risk for CRC. The test had an 87.5% sensitivity for stage I, II, or III colorectal cancer but only a 13% sensitivity for advanced precancerous lesions. Test specificity was 89.6%, meaning that about 1 in 10 participants without CRC or advanced precancerous lesions on colonoscopy had a false-positive result.
Although the Shield blood test has a higher rate of false positives than the traditional fecal immunochemical test (FIT) and lower sensitivity and specificity than a multitarget stool DNA (FIT-DNA) test designed to improve on Cologuard, it meets the previously established criteria set forth by the Centers for Medicare & Medicaid Services (CMS) to be covered for Medicare beneficiaries at 3-year intervals, pending FDA approval.
A big concern, however, is that the availability of a blood test may cause patients who would have otherwise been screened with colonoscopy or stool tests to switch to the blood test. A cost-effectiveness analysis found that offering a blood test to patients who decline screening colonoscopy saves additional lives, but at the cost of more than $377,000 per life-year gained. Another study relying on three microsimulation models previously utilized by the US Preventive Services Task Force (USPSTF) found that annual FIT results in more life-years gained at substantially lower cost than blood-based screening every 3 years “even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.” As a result, a multidisciplinary expert panel concluded that blood-based screening should not substitute for established CRC screening tests, but instead be offered only to patients who decline those tests.
In practice, this will increase the complexity of the CRC screening conversations we have with patients. We will need to be clear that the blood test is not yet endorsed by the USPSTF or any major guideline group and is a second-line test that will miss most precancerous polyps. As with the stool tests, it is essential to emphasize that a positive result must be followed by diagnostic colonoscopy. To addend the cancer screening maxim I mentioned before, the blood test is not the best test for CRC, but it’s probably better than no test at all.
Dr. Lin is a family physician and associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor.
A version of this article appeared on Medscape.com.
Last year, I concluded a commentary for this news organization on colorectal cancer (CRC) screening guidelines by stating that between stool-based tests, flexible sigmoidoscopy, and colonoscopy, “the best screening test is the test that gets done.” But should that maxim apply to the new blood-based screening test, Guardant Health Shield? This proprietary test, which costs $895 and is not generally covered by insurance, identifies alterations in cell-free DNA that are characteristic of CRC.
Shield’s test characteristics were recently evaluated in a prospective study of more than 10,000 adults aged 45-84 at average risk for CRC. The test had an 87.5% sensitivity for stage I, II, or III colorectal cancer but only a 13% sensitivity for advanced precancerous lesions. Test specificity was 89.6%, meaning that about 1 in 10 participants without CRC or advanced precancerous lesions on colonoscopy had a false-positive result.
Although the Shield blood test has a higher rate of false positives than the traditional fecal immunochemical test (FIT) and lower sensitivity and specificity than a multitarget stool DNA (FIT-DNA) test designed to improve on Cologuard, it meets the previously established criteria set forth by the Centers for Medicare & Medicaid Services (CMS) to be covered for Medicare beneficiaries at 3-year intervals, pending FDA approval.
A big concern, however, is that the availability of a blood test may cause patients who would have otherwise been screened with colonoscopy or stool tests to switch to the blood test. A cost-effectiveness analysis found that offering a blood test to patients who decline screening colonoscopy saves additional lives, but at the cost of more than $377,000 per life-year gained. Another study relying on three microsimulation models previously utilized by the US Preventive Services Task Force (USPSTF) found that annual FIT results in more life-years gained at substantially lower cost than blood-based screening every 3 years “even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.” As a result, a multidisciplinary expert panel concluded that blood-based screening should not substitute for established CRC screening tests, but instead be offered only to patients who decline those tests.
In practice, this will increase the complexity of the CRC screening conversations we have with patients. We will need to be clear that the blood test is not yet endorsed by the USPSTF or any major guideline group and is a second-line test that will miss most precancerous polyps. As with the stool tests, it is essential to emphasize that a positive result must be followed by diagnostic colonoscopy. To addend the cancer screening maxim I mentioned before, the blood test is not the best test for CRC, but it’s probably better than no test at all.
Dr. Lin is a family physician and associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor.
A version of this article appeared on Medscape.com.
Last year, I concluded a commentary for this news organization on colorectal cancer (CRC) screening guidelines by stating that between stool-based tests, flexible sigmoidoscopy, and colonoscopy, “the best screening test is the test that gets done.” But should that maxim apply to the new blood-based screening test, Guardant Health Shield? This proprietary test, which costs $895 and is not generally covered by insurance, identifies alterations in cell-free DNA that are characteristic of CRC.
Shield’s test characteristics were recently evaluated in a prospective study of more than 10,000 adults aged 45-84 at average risk for CRC. The test had an 87.5% sensitivity for stage I, II, or III colorectal cancer but only a 13% sensitivity for advanced precancerous lesions. Test specificity was 89.6%, meaning that about 1 in 10 participants without CRC or advanced precancerous lesions on colonoscopy had a false-positive result.
Although the Shield blood test has a higher rate of false positives than the traditional fecal immunochemical test (FIT) and lower sensitivity and specificity than a multitarget stool DNA (FIT-DNA) test designed to improve on Cologuard, it meets the previously established criteria set forth by the Centers for Medicare & Medicaid Services (CMS) to be covered for Medicare beneficiaries at 3-year intervals, pending FDA approval.
A big concern, however, is that the availability of a blood test may cause patients who would have otherwise been screened with colonoscopy or stool tests to switch to the blood test. A cost-effectiveness analysis found that offering a blood test to patients who decline screening colonoscopy saves additional lives, but at the cost of more than $377,000 per life-year gained. Another study relying on three microsimulation models previously utilized by the US Preventive Services Task Force (USPSTF) found that annual FIT results in more life-years gained at substantially lower cost than blood-based screening every 3 years “even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.” As a result, a multidisciplinary expert panel concluded that blood-based screening should not substitute for established CRC screening tests, but instead be offered only to patients who decline those tests.
In practice, this will increase the complexity of the CRC screening conversations we have with patients. We will need to be clear that the blood test is not yet endorsed by the USPSTF or any major guideline group and is a second-line test that will miss most precancerous polyps. As with the stool tests, it is essential to emphasize that a positive result must be followed by diagnostic colonoscopy. To addend the cancer screening maxim I mentioned before, the blood test is not the best test for CRC, but it’s probably better than no test at all.
Dr. Lin is a family physician and associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167815</fileName> <TBEID>0C04FBDD.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FBDD</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>353</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240423T115627</QCDate> <firstPublished>20240423T125432</firstPublished> <LastPublished>20240423T125432</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240423T125432</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Kenneth W. Lin, MD</byline> <bylineText>KENNETH W. LIN, MD, MPH</bylineText> <bylineFull>KENNETH W. LIN, MD, MPH</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>Opinion</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>If public and private payers start covering Shield alongside other CRC screening tests, it presents an opportunity for primary care physicians to reach the appr</metaDescription> <articlePDF/> <teaserImage/> <title>CRC Screening in Primary Care: The Blood Test Option</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>GIHOLD</publicationCode> <pubIssueName>January 2014</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term canonical="true">31</term> <term>21</term> <term>15</term> </publications> <sections> <term canonical="true">52</term> <term>41022</term> </sections> <topics> <term>67020</term> <term canonical="true">280</term> <term>270</term> <term>278</term> <term>263</term> <term>213</term> <term>38029</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>CRC Screening in Primary Care: The Blood Test Option</title> <deck/> </itemMeta> <itemContent> <p>Last year, I concluded a <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/995196">commentary</a></span> for this news organization on <span class="Hyperlink"><a href="https://emedicine.medscape.com/article/2500006-overview">colorectal cancer</a></span> (CRC) screening guidelines by stating that between stool-based tests, <span class="Hyperlink"><a href="https://emedicine.medscape.com/article/1637664-overview">flexible sigmoidoscopy</a></span>, and <span class="Hyperlink"><a href="https://emedicine.medscape.com/article/1819350-overview">colonoscopy</a></span>, “the best screening test is the test that gets done.” But should that maxim apply to the new blood-based screening test, Guardant Health Shield? This proprietary test, which costs $895 and is not generally covered by insurance, <span class="Hyperlink"><a href="https://www.aafp.org/pubs/afp/issues/2023/0600/diagnostic-tests-guardant-health-shield-colon-cancer.html">identifies alterations in cell-free DNA that are characteristic of CRC</a></span>.</p> <p>Shield’s test characteristics were recently evaluated in a <span class="Hyperlink"><a href="https://www.nejm.org/doi/full/10.1056/NEJMe2311101">prospective study</a></span> of more than 10,000 adults aged 45-84 at average risk for CRC. The test had an 87.5% sensitivity for stage I, II, or III colorectal cancer but only a 13% sensitivity for advanced precancerous lesions. Test specificity was 89.6%, meaning that about 1 in 10 participants without CRC or advanced precancerous lesions on colonoscopy had a false-positive result.<br/><br/>Although the <span class="Hyperlink"><a href="https://www.nejm.org/doi/10.1056/NEJMoa2310336?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed">Shield blood test</a></span> has a higher rate of false positives than the traditional fecal immunochemical test (FIT) and lower sensitivity and specificity than a multitarget stool DNA (FIT-DNA) test designed to improve on Cologuard, it meets the <span class="Hyperlink"><a href="https://www.cms.gov/files/document/mm12280.pdf">previously established criteria</a></span> set forth by the Centers for Medicare & Medicaid Services (CMS) to be covered for Medicare beneficiaries at 3-year intervals, pending FDA approval.<span class="tag metaDescription"> If public and private payers start covering Shield alongside other CRC screening tests, it presents an opportunity for primary care physicians to reach the approximately <span class="Hyperlink"><a href="https://www.cdc.gov/pcd/issues/2023/pdf/23_0071.pdf">3 in 10 adults between ages 45 and 75 who are not being routinely screened</a></span>.</span><br/><br/>A big concern, however, is that the availability of a blood test may cause patients who would have otherwise been screened with colonoscopy or stool tests to switch to the blood test. A <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2811942">cost-effectiveness analysis</a></span> found that offering a blood test to patients who decline screening colonoscopy saves additional lives, but at the cost of more than $377,000 per life-year gained. <span class="Hyperlink"><a href="https://www.gastrojournal.org/article/S0016-5085(24)00174-4/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F">Another study</a></span> relying on three microsimulation models previously utilized by the US Preventive Services Task Force (USPSTF) found that annual FIT results in more life-years gained at substantially lower cost than blood-based screening every 3 years “even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.” As a result, a multidisciplinary <span class="Hyperlink"><a href="https://www.cghjournal.org/article/S1542-3565(24)00162-9/abstract">expert panel concluded</a></span> that blood-based screening should not substitute for established CRC screening tests, but instead be offered only to patients who decline those tests.<br/><br/>In practice, this will increase the complexity of the CRC screening conversations we have with patients. We will need to be clear that the blood test is not yet endorsed by the USPSTF or any major guideline group and is a second-line test that will miss most precancerous polyps. As with the stool tests, it is essential to emphasize that a positive result must be followed by diagnostic colonoscopy. To addend the cancer screening maxim I mentioned before, the blood test is not the best test for CRC, but it’s probably better than no test at all.<span class="end"/></p> <p> <em>Dr. Lin is a family physician and associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at <span class="Hyperlink"><a href="https://commonsensemd.blogspot.com/">Common Sense Family Doctor.</a></span></em> </p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/crc-screening-primary-care-blood-test-option-2024a10007gf">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> <p>“The best screening test is the test that gets done,” but should that maxim apply to the new blood-based screening test?</p> </itemContent> </newsItem> </itemSet></root>
